Abstract
There has been considerable interest in the development of dopamine D3 receptor (DRD3) partial agonists and antagonists for the treatment of substance use disorders. Pre-clinical evidence overwhelmingly supports the use of these drugs, but translation to humans has remained elusive due to the lack of selective compounds that are suitable for use in humans. Although it has been established for full antagonists, little in vivo occupancy data are available with DRD3 partial agonists. Here we investigate for the first time in healthy controls, the in vivo occupancy of a novel D3 partial agonist (BP1.4979) at the DRD3 and DRD2. Participants received either a single dose (1, 3, 10 or 30 mg) or a subchronic regimen (5–7 days, q.d. or b.i.d) of BP1.4979, with the last dose given at 1, 12 or 24 h prior to scanning with [11C]-(+)-PHNO. Single and subchronic administration of BP1.4979 dose-dependently occupied the DRD3 and DRD2, and this occupancy was preferential for the DRD3, notably at longer time points after administration of BP1.4979. Also consistent with preference for the DRD3, prolactin levels were minimally increased, and no subjective effects of BP1.4979 were reported. Serum levels of BP1.4979 were higher than its active metabolite, BP1.6239, while no notable increases in the inactive metabolite, BP1.6197, were found. These findings indicate the range of doses that can be used to occupy selectively the DRD3 over the DRD2 with BP1.4979 and speak to the use of in vivo imaging approaches in dose finding studies.
Similar content being viewed by others
Introduction
Dopamine (DA) has been established as important to substance use disorders [1, 2]. Converging evidence implicates the D3 receptor (DRD3) [3] as a target for medications development [4]. The DRD3 shares homology with the DRD2 [5], and efforts have been focussed on developing ligands that are selective for the DRD3 as compared to the DRD2, especially given that administration of DRD2 antagonists can produce debilitating side effects [6, 7]. A number of compounds selective for the DRD3 have been developed [8] and some antagonists have been tested in humans [9,10,11]. It is important that novel drugs be evaluated for their occupancy of the DRD3 in vivo, as otherwise the dose range selected for clinical trial studies may be inadequately selected to study the role of DRD3 [12].
PET imaging is a non-invasive technique that allows for the measurement of receptor occupancy. One agonist tracer, [11C]-(+)-PHNO [13], allows for the measurement of occupancy at both DRD2 and DRD3. Through measurement of binding potentials at different regions of interest, regional fractions of DRD2 or DRD3 levels can be determined [12]. In the elegant work of Tziortizi et al. [14], it was demonstrated that approximately 100% of binding in the substantia nigra (SN) is to the DRD3, 75% of binding in the ventral pallidum (VP) is to the DRD3, while it is 65% in the globus pallidus (GP). These regional fractions are consistent with the animal literature [15, 16] and speak to the validity of using [11C]-(+)-PHNO to measure differentially DRD3 and DRD2.
Imaging with PET and [11C]-(+)-PHNO can provide an important step in the development of DRD3 selective compounds. In our previous study [17], we used this approach to measure occupancy of the DRD3 and DRD2 by buspirone, an antagonist that has been shown, in pre-clinical studies, to have greater affinity for the DRD3 than the DRD2 [18]. In our study, we found that, in humans in vivo, buspirone did not bind more to the DRD3 than the DRD2. This difference in the pre-clinical findings and the clinical data underscores the importance of human in vivo investigations for determination of receptor occupancy levels. Indeed, in a previous study, another antagonist, GSK598809, was administered prior to scanning with [11C]-(+)-PHNO [19]. It was found that GSK598809 displaced [11C]-(+)-PHNO in the SN but not the dorsal caudate (DC), with intermediate displacement in the ventral striatum (VST; also known as the limbic striatum (LST)) and GP. However, it remains to be determined whether a DRD3 partial agonist can selectively occupy the DRD3 as compared to the DRD2. In this regard, a recent investigation studied the occupancy of the DRD3 and DRD2 following the administration of cariprazine to participants with schizophrenia. It was found that this DRD3 partial agonist preferentially occupied the DRD3, over the DRD2, at low doses [20].
The purpose of the present study was to investigate, for the first time in healthy controls, the in vivo occupancy of the DRD3 and DRD2 by a selective DRD3 partial agonist. BP1.4979 has an affinity for the human DRD3 of ~1 nM and presents a partial agonist behaviour with an intrinsic activity of 32% ± 2.6% and EC50 of 0.7 ± 0.3 nM. In contrast, it behaves as an antagonist at the hDRD2 with Ki of 192 nM. After oral administration in humans, it reaches peak serum concentrations in one hour and has a half-life of about 8 h. In the present study, participants were administered a number of acute doses of BP1.4979 one hour prior to PET scanning with [11C]-(+)-PHNO to characterise the occupancy of DRD3 and DRD2. BP1.4979 was then administered at various time points prior to scanning with [11C]-(+)-PHNO to determine the time course of binding of BP1.4979 to the DRD3 or DRD2. Participants then took BP1.4979 at home for approximately 7 days to determine the effects of sub-chronic dosing of BP1.4979, taken either once or twice a day, on binding to the DRD3 or DRD2. Prolactin and BP1.4979 levels, as well 2 metabolites of BP1.4979 (one pharmacologically active: BP1.6239; and one inactive: BP1.6197) were also measured in serum. Subjective ratings and adverse events were also recorded. It was hypothesised that the percent occupancy of the DRD3 regions would be greater than that for the DRD2 regions following administration of BP1.4979. The aim of this study was to explore the dosing and dosing regimen that optimally produced occupancy of the DRD3 regions above the DRD2 regions.
Methods
Participants
All procedures were approved by the Centre for Addiction and Mental Health Research Ethics Board and complied with the 1975 Helsinki Declaration (5th revision, 2000). Participants (>19 years old) were recruited from the community and provided written informed consent. Inclusion criteria is provided in Suppementary Material.
Procedure
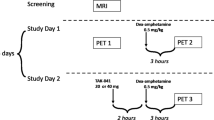
This single-blind study of BP1.4979 was divided into several parts (sample sizes provided in Table S1 in Supplementary Material):
-
1.
Part 1: Dose-response: The first part was a dose-response study of acute doses (0, 1, 3, 10, 30 mg) that were administered one hour prior to the start of the PET scan. Participants (n = 6) received four treatments in fixed order (0, 3, 1 and 10 mg). Three (n = 3) participants received placebo followed by 30 mg on separate days.
-
2.
Part 2: Time course: PET scans were started either 12 h after an acute 10 mg dose (10 mg 12 h, n = 4), or 24 h after acute doses of either 1, 3 or 10 mg (1 mg 24 h, n = 2; 3 mg 24 h, n = 3; 10 mg 24 h, n = 2). Each participant also underwent a [11C]-(+)-PHNO scan after placebo. Some participants partook in more than one condition.
-
3.
Part 3: Subchronic dosing: Participants took BP1.4979 orally at home once a day for approximately 7 days. In 4 participants, the scan was conducted both 1 and 24 h after the last dose of sub-chronic dose of 10 mg (10 mgSC 1 h, 10 mgSC 24 h). Two other participants were scanned 24 h after the last sub-chronic dose of 3 mg (3 mgSC 24 h). Each participant also underwent a [11C]-(+)-PHNO scan after placebo. For doses that occurred at home, participants were called at the time of dosing to remind them to take their pill and also to assess compliance.
-
4.
Part 4: B.I.D. dosing: Based on the results of the first 3 parts, it was decided that B.I.D. administration may be preferable to achieve lasting and preferential occupancy of the D3 receptors. Thus, in the fourth part of the study, participants took BP1.4979 orally at home twice a day for 5-7 days at either 5 mg B.I.D., 10 mg B.I.D. or 15 mg B.I.D. (n = 4 each group), and were scanned both at 1 h (5mgBID 1 h, 10mgBID 1 h, 15mgBID 1 h) and 12 h (5mgBID 12 h, 10mgBID 12 h, 15mgBID 12 h) after the last dose. Each participant also underwent a [11C]-(+)-PHNO scan after placebo.
To control for absorption of the drug, participants were asked to fast for at least 3 h prior to attending the lab and were given a light snack about 90 min prior to the start of the scan.
During each PET session, 4 blood samples were taken for analysis of serum levels of prolactin, BP1.4979 and metabolites of BP1.4979. The first was taken one hour prior to the start of the scan (to correspond with the time of immediately before dosing). After that, 3 blood samples were taken at one hour intervals. Visual analog scales (VAS; items are reported in the Supplementary Material) were given at baseline (pre-dosing) and at every hour following that. Adverse events were assessed at each visit and for each of the two days following the scans (see Supplementary Material).
Analysis of BP1.4979 and its metabolites (BP1.6239 and BP1.6197)
PET image analysis
Region of interest (ROI)-based analysis
ROI delineation and time activity curve analyses were performed using ROMI (details in ref. [21]). Functional sub-compartments of the striatum [22] including the associative striatum (AST), limbic striatum (LST), and sensorimotor striatum (SMST) were chosen as ROIs. Delineation for the (whole) VP and SN is described elsewhere [23]. The globus pallidus (GP) was delineated in ROMI.
Binding potential:
[11C]-(+)-PHNO specific binding potential (BPND) was estimated in each ROI using the simplified reference tissue method [24] (SRTM), with cerebellar cortex (excluding vermis) as reference region. Parameter estimation was performed using PMOD (Version 2.8.5; PMOD Technologies Ltd, Zurich, Switzerland).
DRD3 vs. DRD2 component:
As we are interested in the DRD3 vs. DRD2 selectivity of BP1.4979 we estimated the overall average DRD3 component (BPD3’) and DRD2 component (BPD2’). To calculate baseline BPD3’, BPND in each ROI (SN, VP, GP, LST, AST, SMST) was multiplied by established regional fractions [14] (fD3(SN) = 1.0; fD3(VP) = 0.75; fD3(GP) = 0.65; fD3(LST) = 0.26; fD3(SMST) = 0.02; fD3(AST) = 0). The product of BPND and fD3 from each of the 6 ROIs was then averaged to derive BPD3’ at baseline. To calculate baseline BPD2’, the same equation was applied except that the BPND of ROIs was multiplied by (1-fD3). Under blocking conditions, BPD3’ and BPD2’ were the averages of regional BPNDBP1.4979[D3] and BPNDBP1.4979[D2] calculated with Eqs 4 and 5 (see below), respectively, i.e., using modified fD3 accounting for drug occupancy.
Occupancy
For ROIs, percent occupancy was defined as the percentage reduction in BPND from 0 mg (placebo) scan to the BP1.4979 exposed state, calculated for each participant using the following Eq. 1:
To obtain percentage occupancy at DRD3 and DRD2 sites for a blocking scan, assuming constant %Occupancy[D3] and %Occupancy[D2] across all regions, a six-point (6 ROIs) linear regression was performed by using Eq. 2:
This equation was derived from Eqs. 1 and 3–5:
The model constraints were 0 < %Occupancy[D2] < 100, 0 < %Occupancy[D3] < 100 and %Occupancy[D3] > %Occupancy[D2], given the observed DRD3 preference of BP1.4979. The linear regressions were performed in GraphPad Prism software.
Data analyses
Differences in DRD3 and DRD2 component were analysed with repeated-measures Dose/Condition × Component (2 levels; BPD3’, BPD2’) ANOVAs. Significant ANOVAs were followed by Bonferroni-corrected paired t-tests of placebo to each Dose/Condition. %Occupancy was analysed with %Occupancy (2 levels; %Occupancy[D3], %Occupancy[D2]) × Dose/Condition ANOVAs followed by Bonferroni-corrected paired t-tests of %Occupancy[D3] to %Occupancy[D2] for each Dose/Condition. For all analyses, sphericity was assessed with the Mauchley’s test and criterion for significance was set at p < 0.05. Analyses were conducted with SPSS (version 24).
Results
Participants were 28 non-smokers (20 male; 16 Caucasian, 5 Asian, 2 Hispanic, 2 Mixed, 3 African American). The mean ± SEM age was 40 ± 2.65 and all were within normal body mass index (24.27 ± 0.51).
Adverse events
BP1.4979 was generally well-tolerated. For a description of adverse events and subjective effects, see Supplementary Material.
See Table S1 (Supplemental Material) for a breakdown of sample sizes in the various conditions.
Part 1: acute dose-response
BPD2’ and BPD3’ (Fig. 1): Analysis of the differences in BPD3’ and BPD2’ component with a repeated-measures Component (2 levels; BPD2’, BPD3’) × Dose (4 levels; 0, 1, 3, 10; the 30 mg dose is not included in the ANOVA because it consisted of a different group of participants) ANOVA revealed a significant interaction (F(3,15) = 8.454, p = 0.002; n = 6), indicating that the effect of BP1.4979 dose-dependently decreases [11C]-(+)-PHNO binding differently in the BPD3’ and the BPD2’ (Fig. 1). Bonferroni-corrected t-tests found that, for the BPD3’, placebo was different from the 3 and 10 mg doses, and for the BPD2’, placebo was different from the 10 mg dose (n = 6; adjusted p value of 0.0167). In a separate ANOVA for the 30 mg dose (n = 3), the Component (2 levels; BPD2’, BPD3’) × Dose (2 levels; 0, 30) interaction was not significant (p > 0.1), but there was a significant effect of Dose (F(1, 2) = 25.721. p = 0.037) and Component (F(1,2) = 55.859, p < 0.017), suggesting that BP1.4979 decreased BPD2’ and BPD3’ to the same extent. t-Tests on the difference between placebo and the 30 mg dose, separately for each of the BPD3’ and BPD2,’ revealed significant effects for both of these comparisons (p < 0.05).
Dose-response study of acute doses of BP1.4979 administered one hour prior to PET scanning with [11C]-(+)-PHNO. Left panel: BPD2’ and BPD3’ after administration of acute doses (1, 3, 10 mg: n = 6; 30 mg: n = 3). Grey and open symbols represent BPD3’ and BPD2’, respectively; black symbols represent placebo condition for the 30 mg dose group. *p < 0.05, different from placebo for either BPD2’ or BPD3’. Right panel: Mean ± SEM %Occupacny of the DRD3 (grey sumbols) or DRD2 (open symbols). *p < 0.05, %Occupancy[D3] vs. %Occupancy[D2]
%Occupancy: Analysis of %Occupancy with a %Occupancy (2 levels; %Occupancy[D3], %Occupancy[D2])] × Dose (3 levels; 1, 3, 10 mg) ANOVA on the dose-response revealed a significant interaction (F(2, 10) = 9.427, p = 0.005), indicating that the effect of Dose is different for %Occupancy[D3] or %Occupancy[D2]. Bonferroni-corrected t-tests revealed that %Occupancy[D3] is different from %Occupancy[D2] at the 3 and 10 mg doses (adjusted p value of 0.0167). Separate Bonferroni-corrected t-tests on the 30 mg dose revealed that %Occupancy[D3] and %Occupancy[D2] were different (p < 0.05).
Part 2: time course analysis
BPD2’ and BPD3’ (Fig. 2): Analysis of the differences in BPD3’ and BPD2’ component with repeated-measures Component (2 levels; BPD2’, BPD3’) × Condition (2 levels; placebo vs. 3 mg 24 h or 10 mg 12 h) ANOVAs revealed a significant interaction for 10 mg 12 h condition (F(1, 3) = 30.925, p = 0.011), and only an effect of Component for the 3 mg 24 h Condition (n = 3; F1, 2) = 19.773, p = 0.047). Bonferroni-corrected paired t-tests suggested that the 10 mg 12 h (n = 4) Condition was significantly different from placebo for the BPD3’ (p = 0.023; adjusted p value of 0.025) but not the BPD2’ (p = 0.132; adjusted p value of 0.025); no significant effects for the 3 mg 24 h Condition were revealed by Bonferroni-corrected t-tests. For the 1 mg 24 h and 10 mg 24 h Conditions, the small sample sizes (n = 2) precluded statistical analyses. Data is provided for visual inspection in Fig. 2.
Time course study of several acute doses (1, 3 and 10 mg) of BP1.4979 administered at various times (12 or 24 h) prior to PET scanning with [11C]-(+)-PHNO. Left panel: BPD2’ and BPD3’ after administration of acute doses (10 mg 12 h, n = 4; 10 mg 24 h, n = 2; 1 mg 24 h, n = 2; 3 mg 24 h n = 3). Grey and open symbols represent BPD3’ and BPD2’, respectively. Black symbols represent the placebo condition for 3 mg 24 h group. Placebo conditions not shown for 1 mg 24 h and 10 mg 24 h conditions because the small sample sizes precluded analyses. *Different from placebo for the BPD3’ (p < 0.05). Right panel: Mean ± SEM percent occupancy of the DRD3 (grey sumbols) or DRD2 (open symbols). *p < 0.05, %Occupancy[D3] vs. %Occupancy[D2]
%Occupancy: Analysis of occupancy with ANOVAs was precluded due to the single condition in the 3 mg 24 h or 10 mg 12 h groups, but Bonferroni-corrected t-tests revealed that the %Occupancy[D2] was different than the %Occupancy[D3] at the 10 mg 12 h condition, but not the 3 mg 24 h condition (adjusted p value of 0.025). For the 1 mg 24 h and 10 mg 24 h Conditions, the small sample sizes (n = 2) precluded statistical analyses. Data is provided for visual inspection in Fig. 2.
Part 3: sub-chronic dosing
BPD2’ and BPD3’ (Fig. 3): Analysis of the differences in BPD3’ and BPD2’ component with a repeated-measures Component (2 levels; BPD2’, BPD3’) × Condition (3 levels; placebo, 10 mgSC 1 h, 10 mgSC 24 h) ANOVA revealed a significant interaction (F(2, 6) = 5.696, p = 0.041, n = 4). Bonferroni-corrected t-tests of each Condition to placebo revealed that placebo was different from 10 mgSC 1 h, but not 10 mgSC 24 h, for fD3’ and fD2’ (adjusted p value of 0.025). For the 3 mgSC 24 h Condition, the small sample size (n = 2) precluded analysis, but data is provided in Fig. 3 for visual inspection.
Study of once daily subchronic doses of BP1.4979, with the last dose administered one hour or 24 h prior to PET scanning with [11C]-(+)-PHNO. Left panel: BPD2’ and BPD3’ after administration of the acute doses (10 mgSC 1 h, n = 4; 10 mgSC 24 h, n = 4; 3 mgSC 24 h, n = 2). Grey and open symbols represent BPD3’ and BPD2’, respectively. Placebo not shown for 3 mg 24 h because the small sample size precluded analyses. *p < 0.05, different from placebo for either BPD2’ or BPD3’. Right panel: Mean ± SEM percent occupancy of the DRD3 (grey sumbols) or DRD2 (open symbols). *p < 0.05, %Occupancy[D3] vs. %Occupancy[D2]
%Occupancy: Analysis of occupancy with a %Occupancy (2 levels; %Occupancy[D2], %Occupancy[D3]) × Condition (2 levels; 10 mgSC 1 h, 10 mgSC 24 h) ANOVA did not reveal an interaction, but an effect of %Occupancy was found (F(1, 3) = 42.274, p = 0.007), suggesting that %Occupancy[D2] and %Occupancy[D3] were different. Bonferroni-corrected t-tests revealed that %Occupancy[D2] was different from %Occupancy[D3] for the 10 mgSC 1 h condition (adjusted p value of 0.025), but not the 10 mgSC 24 h condition. Analysis of the 3 mgSC 24 h condition was precluded due to the small sample size. Data are presented for visual inspection.
Part 4: B.I.D. administration
BPD2’ and BPD3’ (Fig. 4): Analysis of the differences in BPD3’ and BPD2’ component with a mixed Component (2 levels; BPD2’, BPD3’) × Condition (3 levels; placebo, 1, 12 h) × Dose (3 levels; 5mgBID, 10mgBID, 15mgBID) ANOVA with Dose as the between-subjects factor revealed a three-way interaction (F(4, 18) = 10.853, p = <0.001; n = 4 each group), suggesting that the effects of BP1.4979 were different in BPD2’ and BPD3’ at the various time points after treatment and that this varied by dose. Follow-up analysis with Bonferroni-corrected t-tests revealed that the 1 h condition was different from placebo for each of the BPD2’ and the BPD3’ for all doses (adjusted p value of 0.0125). Placebo was also different from the 12 h condition for BPD3’ for the 10mgBID and 15mgBID doses (adjusted p value of 0.0125). For the BPD2’, the 12 h condition was different from placebo for the 10mgBID dose (adjusted p value of 0.0125).
Study of B.I.D. (twice daily) subchronic doses of BP1.4979, with the last dose administered one hour or 12 h prior to PET scanning with [11C]-(+)-PHNO. Left panel: BPD2’ and BPD3’ after administration of the acute doses (n = 4 for each dose). Grey and open symbols represent BPD3’ and BPD2’, respectively. Open, grey and dark symbols at placebo conditions represent the 5, 10 and 15 mg doses, respectively. *p < 0.05, different from placebo for either BPD2’ or BPD3’. Right panel: Mean ± SEM percent occupacny of the DRD3 (grey sumbols) or DRD2 (open symbols). *p < 0.05, %Occupancy[D3] vs. %Occupancy[D2]
%Occupancy
Analysis of occupancy with a %Occupancy (%Occupancy[D2], %Occupancy[D3]) X Condition (1, 12 h) ANOVA separately for each of the 5, 10 or 15 mg doses revealed an effect of %Occupancy (F(1, 3) = 14.748, p = 0.031) and Condition (F(1, 3) = 24.289, p = 0.016) for the 5 mg dose and also effects of %Occupancy (F(1, 3) = 169.858, p = 0.001) and Condition (1, 3) = 49.820, p = 0.006) for the 10 mg dose. For the 15 mg dose, a significant interaction was revealed (F(1, 3) = 14.860, p = 0.031). This indicates that %Occupancy[D3] was greater than %Occupancy[D2] for the 5 and 10 mg doses (with Occupancy being greater in the 1 h as compared to the 12 h condition), and that the condition had an effect on this difference for the 15 mg dose. Bonferroni-corrected t-tests revealed that %Occupancy[D2] was different from %Occupancy[D3] at all conditions and doses, except when participants were scanned 12 h after the last dose of 5 mg B.I.D. (adjusted p value of 0.025)
%Occupancies for various ROIs are provided in Supplementary Material, Table S2.
Scan parameters are provided in Supplementary Material, Table S3.
Analyses of prolactin, BP1.4979, BP1.639 and BP1.6197 are provided in Supplementary Material (Tables S4-S7).
Discussion
The purpose of the present study was to investigate, for the first time in healthy participants, the binding of a DRD3 partial agonist to DRD2 and DRD3. It was found, with PET imaging with [11C]-(+)-PHNO, that BP1.4979 occupied the DRD3 more than the DRD2, consistent with a previous report [20]. Compared to control conditions, [11C]-(+)-PHNO binding was decreased when participants were scanned 1 h after administration of BP1.4979; at 1 h after administration of BP1.4979, occupancy of the DRD3 was higher than the DRD2. When scanned at various times after administration of BP1.4979, there was residual occupancy of the DRD3 at 12 h post-dose, most notably following B.I.D. administration. Also after 12 h, [11C]-(+)-PHNO binding was decreased more at the DRD3. Inspection of occupancy data suggests that BP1.4979 may occupy the DRD3 more than the DRD2 when participants were scanned 24 h after being given BP1.4979. Changes in [11C]-(+)-PHNO binding and percent occupancy showed region-dependent changes, with greater effects of BP1.4979 in areas with higher fD3. Change in [11C]-(+)-PHNO binding and percent occupancy were more long-lasting than measured increases of serum levels of BP1.4979 or its active metabolite, BP1.6239, and this was most evident after B.I.D. administration. There were small, non-significant increases in prolactin at most doses tested. There was no change in subjective ratings following administration of BP1.4979. BP1.4979 was well-tolerated.
In the present study, dose-dependent decreases in [11C]-(+)-PHNO binding in the BPD3’ and BPD2’ were observed. These decreases were significant for the BPD3’ when 3, 10 and 30 mg were administered one hour prior to scanning; for the BPD2’, these changes were significat after 10 and 30 mg doses. When single acute doses were administered 12 or 24 h prior to PET scanning with [11C]-(+)-PHNO, significant decreases were observed only in only the BPD3’ 12 h after administration of BP1.4979. When 10 mg was administered subchronically once daily, BPD2’ and BPD3’ were decreased at one hour after the last dose, but not at 24 h, but residual occupancy was noted for the DRD3. By contrast, B.I.D. administration produced long-lasting changes in regional fraction that were evident at 12 h after the last dose following 10 and 15 mg B.I.D. administration in BPD3’ and BPD2’; occupancies were greater in BPD3’ 12 h after dosing. Thus, administration of BP1.4979 at 12 h intervals seems to produce the most long-lasting changes at the DRD3 (and DRD2).
Even though [11C]-(+)-PHNO binding was decreased in both the BPD2’ and BPD3’, the percent occupancy of the DRD3 was higher than that for the DRD2 following administration of BP1.4979. Differences in occupancy of the DRD3 and DRD2 were observed at all doses, but the selectivity was greatest below 30 mg and above 1 mg, with the best relative occupancy being observed at 10 mg, determined by the magnitude of separation between DRD2 and DRD3 separation (BPD3’: 80%; BPD2’: 32%). It should be noted that it is not clear if there is any therapeutic benefit to this separation in occupancy. Time course analysis further revealed that occupancy of the DRD3 was more long-lasting than for the DRD2, with the 24 h pre-treatment time being associated with the best lasting occupancy of the DRD3 (40%; DRD2: 1%). Interesting effects were seen, however, during B.I.D. dosing. Under this regimen, persistent occupancy of the DRD3 was seen 12 h after the last dose (10 mg B.I.D: 61%; 15 mg BID: 83%), where this was not observed to nearly the same extent for the DRD2 (10 mg B.I.D: 8%; 15 mg B.I.D.: 15%). Thus, it appears that 10 mg B.I.D. and 15 mg B.I.D. seem to be the best dosing regimens for achieving lasting occupancy of the DRD3 as compared to the DRD2.
The DRD2 an DRD3 component approach allows for the quantification of the amount of occupancy of the DRD3 relative to the DRD2. Inspection of the changes in relative occupancies in various ROIs provides validation for this approach. The degree of change in occupancy in the various ROIs was greatest in the D3-rich area of the SN, where approximately 100% of the signal is due to DRD3, while the change in regional fraction became progressively smaller in ROIs with more of a D2 signal (AST, SMST). These findings suggest that BP1.4979 is more potent in the D3-rich areas and also occupies the DRD3 more selectively than the DRD2.
Prolactin is released by the pituitary gland and is under inhibitory control of DA. Blockade of the DRD2 releases this inhibition. Increased prolactin (hyperprolactinemia) is a known side effect of treatment with agents that block the DRD2, and thus prolactin levels provide a general estimate of the efficacy of a treatment in blocking the DRD2. In a review of studies on the effects of antipsychotics on prolactin levels, consensus values for estimates of hyperprolactinemia were in the range of about 20-30 ng/ml [25]. Thus, in the present study, good selectivity at the DRD3 is supported by the further finding that prolactin levels were not greatly affected by any dose or pre-treatment regimen with the exception of the highest doses of the acute 30 mg dose and the sub-chronic 15 mg B.I.D. dosing.
Selective effects of BP1.4979 on the DRD3 as opposed to the DRD2 are consistent with the pre-clinical literature that the debilitating side effects of treatments with dopamine antagonists are related to actions at the DRD2 [12]. Indeed, in the present study, BP1.4979 was well-tolerated. This is consistent with the pre-clinical evidence that the effects of D3 ligands on behaviour are fairly selective and devoid of any off-target events [26]. It should be noted that, in the present study, participants were also undergoing scanning procedures and thus there is some ambiguity as to whether some adverse events recorded were due to the scanning procedure. Regardless, ratings on the VAS were not affected and all adverse events were mild in intensity.
BP1.4979 has two main metabolites: BP1.6239 and BP1.6197, the former being an active metabolite. In the present study, no appreciable increases in BP1.6197 were observed. By comparison, levels of BP1.4979 and BP1.6239 were increased in a manner that is consistent with their known pharmacokinetics (half life of 8 h and reaching peak values within one hour). What was surprising is that the occupancy of the DRD3 was more long-lasting than the elevations in serum levels of BP1.4979. This is most apparent in consideration of the B.I.D. dosing regimen, where BP1.4979 and BP1.6239 were negligible at 12 h after the last dosing under 10 mg B.I.D. or 15 mg B.I.D., but the occupancy of the DRD3 remained at 61% and 83%, respectively. This suggests that the elimination of the drug from the brain has different pharmacokinetics than it does systemically. This observation warrants further exploration and speaks to the importance of using in vivo PET imaging in dose finding studies, especially for drugs that are believed to have psychotropic effects.
In summary, BP1.4979, and DRD3 partial agonists, warrant further exploration as treatment approaches that target the DRD3. Based on the results of the present study, a B.I.D. dosing regimen may be optimal for achieveing long-lasting occupancy of the DRD3, even after it has cleared from the plasma. More specifically for BP1.4979, it appears that 10 mg B.I.D. or 15 mg B.I.D. may have the best persistent occupancy of the DRD3. However, 15 mg B.I.D. also increased prolactin levels, and thus 10 mg B.I.D. may be the optimal dose for further testing.
Limitations
This study is not without limitations. Briefly: (1) The sample size in this study was small. This limits generalisability of the results somewhat and may also explain the lack of power in some of the analyses. Despite this, effects were quite apparent and the interpretation of the results would likely not be changed with a larger sample size; (2) This study is underpowered to observe effects of gender [27]; (3) The total (combined labelled and unlabelled) mass injected was lower for the 10 mg dose as compared to placebo in the acute dose study. However, the direction of change in effect is not the same as that which would be predicted by the difference in mass. Thus, the differences in mass injected likely did not have any effect in the present study. This is supported by the further findings that the 10 mg dose produced comparable changes in regional fraction in other parts of this study where the mass injected was not different from placbeo; and (4) SRTM is known to underestimate BPND in regions of high binding [28]. However, this effect seems to be negligible as shown in previous [11C]-(+)-PHNO PET occupancy studies (Girgis et al. [19, 29]).
Conclusions
The present study is the first to investigate, in healthy participants, the in vivo occupancy of DRD2 or DRD3 by a DRD3 partial agonist. BP1.4979 had greater occupancy of DRD3 as opposed to DRD2, consistent with findings that prolactin levels were not significantly affected at most doses and that subjective ratings were not altered at any dose. These findings are consistent with converging pre-clinical evidence that targeting the DRD3, as opposed to the DRD2, can provide a therapeutic target for the development of treatments for substance dependence. They also point to a critical role of in vivo occupancy in dose finding for new therapeutic targets.
Funding and disclosure
J-CS is co-founder, shareholder and Scientific manager at Bioprojet. BLF received funding from Bioprojet for this project. The remaining authors have nothing to declare.
References
Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17.
Di Chiara G. The role of dopamine in drug-abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137.
Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–51.
Heidbreder C. Rationale in support of the use of selective dopamine D(3) receptor antagonists for the pharmacotherapeutic management of substance use disorders. Naunyn Schmiede Arch Pharmacol. 2013;386:167–76.
Sibley DR, Monsma FJ. Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13:61–69.
Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. J Pharmacol Exp Ther. 2010;334:556–65.
Millan MJ, Dekeyne A, Rivet JM, Dubuffet T, Lavielle G, Brocco M. S33084, a novel, potent, selective, and competitive antagonist at dopamine D(3)-receptors: II. Functional and behavioral profile compared with GR218,231 and L741,626. J Pharmacol Exp Ther. 2000;293:1063–73.
Pich EM, Collo G. Pharmacological targeting of dopamine D3 receptors: possible clinical applications of selective drugs. Eur Neuropsychopharmacol. 2015;25:1437–47.
Mugnaini M, Iavarone L, Cavallini P, Griffante C, Oliosi B, Savoia C, et al. Occupancy of brain dopamine d(3) receptors and drug craving: a translational approach. Neuropsychopharmacology. 2013;38:302–12.
Nathan PJ, O’Neill BV, Mogg K, Bradley BP, Beaver J, Bani M, et al. The effects of the dopamine D(3) receptor antagonist GSK598809 on attentional bias to palatable food cues in overweight and obese subjects. Int J Neuropsychopharmacol. 2012;15:149–61.
Dodds CM, O’Neill B, Beaver J, Makwana A, Bani M, Merlo-Pich E, et al. Effect of the dopamine D(3) receptor antagonist GSK598809 on brain responses to rewarding food images in overweight and obese binge eaters. Appetite. 2012;59:27–33.
Le Foll B, Wilson AA, Graff A, Boileau I, Di Ciano P. Recent methods for measuring dopamine D3 receptor occupancy in vivo: importance for drug development. Front Pharmacol. 2014;5:161.
Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–60.
Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–77.
Kiss B, Horti F, Bobok A. In vitro and in vivo comparison of [(3)H](+)-PHNO and [(3)H]raclopride binding to rat striatum and lobes 9 and 10 of the cerebellum: a method to distinguish dopamine D(3) from D(2) receptor sites. Synapse. 2011;65:467–78.
Rabiner EA, Slifstein M, Nobrega J, Plisson C, Huiban M, Raymond R, et al. In vivo quantification of regional dopamine-D3 receptor binding potential of (+)-PHNO: Studies in non-human primates and transgenic mice. Synapse. 2009;63:782–93.
le Foll B, Payer D, Di Ciano P, Guranda M, Nakajima S, Tong J, et al. Occupancy of dopamine D3 and D2 receptors by buspirone: A [11C]-(+)-PHNO PET study in humans. Neuropsychopharmacology. 2015;41:529–37.
Bergman J, Roof RA, Furman CA, Conroy JL, Mello NK, Sibley DR, et al. Modification of cocaine self-administration by buspirone (buspar(R)): potential involvement of D3 and D4 dopamine receptors. Int J Neuropsychopharmacol. 2013;16:445–58.
Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, et al. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 2010;68:392–9.
Girgis RR, Slifstein M, D’Souza D, Lee Y, Periclou A, Ghahramani P, et al. Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [(11)C]-(+)-PHNO. Psychopharmacology. 2016;233:3503–12.
Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300.
Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. J Neurosci. 2012;32:1353–9.
Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8.
Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. 2014;28:421–53.
Reavill C, Taylor SG, Wood MD, Ashmeade T, Austin NE, Avenell KY, et al. Pharmacological actions of a novel, high-affinity, and selective human dopamine D(3) receptor antagonist, SB-277011-A. J Pharmacol Exp Ther. 2000;294:1154–65.
Cosgrove KP, Wang S, Kim SJ, McGovern E, Nabulsi N, Gao H, et al. Sex differences in the brain’s dopamine signature of cigarette smoking. J Neurosci. 2014;34:16851–5.
Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, et al. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab 2007;27:857–71.
Graff-Guerrero A, Redden L, Abi-Saab W, Katz DA, Houle S, Barsoum P, et al. Blockade of [11C](+)-PHNO binding in human subjects by the dopamine D3 receptor antagonist ABT-925. Int J Neuropsychopharmacol. 2010;13:273–87.
Acknowledgements
The authors wish to thank Dr. Dina Lagzdins for providing medical coverage of PET scans.
Author contributions:
PDC oversaw the day-to-day conduct of the study, analysed the data and wrote the first draft of the manuscript. EM conducted part of the study and PET analyses. JT assisted with the data analyses and interpretation, AAW oversaw the radioligand synthesis, SH oversaw the PET imaging, IB provided oversight for imaging analysis and contributed to interpretation of the data results. TD provided assistance with regulatory issues and drug related issues and study design, PR oversaw analyses of BP1.4979 and metabolites. JCS provided designed the study and interpretation of the data. BLF provided oversight of all study component as Principal Investigator and Qualified investigator. He designed the study. All authors participated in manuscript writing. J-C.S. is co-founder, shareholder and Scientific manager at Bioprojet, B.L.F. received funding from Bioprojet for this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Di Ciano, P., Mansouri, E., Tong, J. et al. Occupancy of dopamine D2 and D3 receptors by a novel D3 partial agonist BP1.4979: a [11C]-(+)-PHNO PET study in humans. Neuropsychopharmacol. 44, 1284–1290 (2019). https://doi.org/10.1038/s41386-018-0285-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-018-0285-4
This article is cited by
-
SLIC-Occ: functional segmentation of occupancy images improves precision of EC50 images
EJNMMI Physics (2023)
-
Effect of scan-time shortening on the 11C-PHNO binding potential to dopamine D3 receptor in humans and test–retest reliability
Annals of Nuclear Medicine (2023)
-
EC50 images, a novel endpoint from PET target occupancy studies, reveal spatial variation in apparent drug affinity
European Journal of Nuclear Medicine and Molecular Imaging (2022)
-
D3 dopamine receptors and a missense mutation of fatty acid amide hydrolase linked in mouse and men: implication for addiction
Neuropsychopharmacology (2020)