Abstract
The most recent update to the International Myeloma Working Group consensus criteria places a strong emphasis on the need for more sensitive haematological markers of response driven by the success of novel therapies. One such marker is serum free light chain (sFLC) analysis, which was first incorporated into the definition of stringent complete response in 2006. However, over the past decade there has been some reluctance to extend the role of the sFLC assays to replace 24 h urine electrophoresis for monitoring multiple myeloma (MM). In this review, we lay out the evidence in favour of serum over urine for monoclonal FLC measurements and propose modified criteria for response assignment in myeloma.
Similar content being viewed by others
Introduction
Monoclonal free light chains (FLCs) in multiple myeloma (MM) patients have historically been monitored in 24 h urine by electrophoretic measurements of Bence Jones protein (BJP), however, much controversy has arisen surrounding the adequacy of this approach for the assessment of FLC response. Limited analytical sensitivity [1, 2], the impact of renal metabolism [3,4,5], and poor provision of urine samples [6,7,8,9] limit the usefulness of urinalysis for monitoring MM patients.
In this context, Freelite® polyclonal immunoassays, which sensitively measure monoclonal serum FLCs (sFLCs) [10], are recommended for the diagnostic work-up of MM [11] and have been included as a biomarker of malignancy in myeloma guidelines [12]. Mounting evidence also suggests that sFLC measurements may be better suited than 24 h urine for monitoring response to therapy. However, except for patients with non-measurable M-protein levels in serum (<10 g/L by SPE) and urine (BJP<200 mg/24 h by UPE), current myeloma guidelines favour urine assessment for monitoring monoclonal FLCs [13,14,15].
This debate has gathered momentum over the last few years, coinciding with the arrival of clinical and laboratory advances in the early 2000s. Immunomodulatory drugs (IMiDs) and proteasome inhibitors have changed the treatment landscape of MM in recent years by inducing high rates of deep, durable responses [16]. More effective treatments have brought about a clinical need for more sensitive monitoring of monoclonal proteins. Since the last guidelines on serum FLC assessment in MM were published by the International Myeloma Working Group (IMWG) nearly a decade ago [11], a number of publications have addressed the analytical performance and clinical contribution of serum FLC immunoassays over urine analyses in the era of novel agents. Here we discuss the merits of sFLCs measurements for monitoring, and propose modified criteria that incorporate sFLC in place of 24 h urine for response assignment in MM patients.
Measuring monoclonal FLCs
The concentration of FLCs in serum reflects the balance between rates of production by plasma cells and clearance by the kidneys. Under normal circumstances, FLCs are rapidly removed from serum and metabolised in the proximal tubules of nephrons. Kidneys can metabolise FLCs in quantities far exceeding production, therefore in healthy individuals FLCs are unlikely to be detected in urine by electrophoretic methods. By contrast over 95% intact immunoglobulin MM (IIMM) and, by definition, all light chain MM (LCMM) patients, produce monoclonal FLCs [17, 18]. Large amounts of monoclonal FLCs must be secreted into the serum before the reabsorptive capacity of the tubules is overwhelmed, and BJP can appear in the urine by overflow proteinuria.
Renal metabolism makes 24 h urine BJP measurements in MM patients unreliable [3, 4]; particularly when monoclonal FLC production is low, as typically seen in patients who respond well to treatment. By contrast, Freelite immunoassays quantify FLCs in serum to levels below 1.0 mg/L, hence providing sensitivity many times greater than that of electrophoretic techniques [19]. A direct consequence of renal metabolism is that absolute measurements of FLC in urine and serum show insufficient correlation and cannot be considered interchangeable [18, 20,21,22]; this has undoubtedly been the main determinant for keeping historic 24 h urine BJP measurements for response assignment in current myeloma guidelines [11].
Clinical value of serum FLC measurements for monitoring
Despite insufficient analytical correlation between serum FLC and 24 h urine, many comparative studies have revealed a clinical benefit of serum measurements for monitoring monoclonal FLC, based on three supporting arguments: (1) superior sensitivity for identifying disease; (2) prognostic value during monitoring; and (3) as an early marker of progression (Fig. 1).
Greater sensitivity of serum FLC measurements for monitoring
As early as 2003, a large study in myeloma patients treated with non-intensive therapy from the UK MRC V–VII trials (1983–1999) reported a far lower rate of LCMM patients achieving a complete response (CR), as determined by normalisation of the sFLC ratio (11%), compared with urinalysis (32%) [18]. Importantly about 10% IIMM patients from the same trial and receiving the same treatment achieved a serological CR, which is comparable to the frequency seen in LCMM using sFLC analysis. These results suggested that 24 h urine measurements lacked sensitivity to reflect tumour burden following therapy, and overestimated the response in a substantial proportion of patients. Analogous results were subsequently reported by the Intergroupe Francophone Du Myélome (IFM) in two independent studies including patients from the IFM2007 [23] and IFM2009 [24] trials who were treated with novel agents. The latter study focused on the responses achieved by patients at the end of VRD (bortezomib, lenalidomide and dexamethasone) induction therapy. At this time, just over 50% of LCMM and IIMM patients had achieved a serological CR as determined by sFLC and SPE assessment, respectively [24]; by contrast 24 h urine was normal in 79% of LCMM patients.
Additionally, trend analyses of patients monitored during treatment indicate that sFLC measurements do not always correlate with urinary BJP measurements over time. In many cases serum measurements are more sensitive and their evolution is consistent with clinical events [5, 23].
Prognostic value of sFLC during monitoring
Several studies have investigated the association between sFLC measurements during monitoring and patient outcome. The majority demonstrate that normalisation of sFLC levels and ratio after treatment associate with improved outcomes, both in LCMM [25] and IIMM [26] patients.
In newly diagnosed LCMM patients treated with novel therapies, the IFM demonstrated that elevated iFLC levels or an abnormal FLC ratio after induction therapy significantly associated with shorter PFS; by contrast UPE and urine immunofixation (uIFE) had no impact on outcomes [27]. Likewise, in 169 LCMM patients from the GEM/PETHEMA clinical trials, sFLC assays had greater sensitivity than UPE for monitoring low levels of disease in certain cases; and those patients whose FLC ratio remained abnormal or their involved FLC levels elevated after treatment had an increased risk of progression [28].
Paiva was the first to show, in a small study of elderly (>65 years), non-transplant eligible patients, that those achieving a stringent CR (sCR) and an immunophenotypic response (determined by flow cytometry) had superior PFS outcomes than those with an immunophenotypic response but not in sCR; although statistical significance was not reached, possibly owing to small sample size and an unconventional categorisation of sCR patients [29]. Subsequently Kapoor assessed the prognostic value of sCR in a prospective study of a combined 445 LCMM and IIMM patients undergoing autologous stem cell transplantation. Patients attaining sCR post-transplant had a distinctive survival advantage over those achieving a CR [26]; hence supporting the inclusion of sCR as a response category in IMWG guidelines [14, 15]. In a later report, Moustafa showed that normalisation of the sFLC ratio retains its prognostic significance throughout monitoring, independent of depth of response [30].
sFLCs as biomarkers of relapse
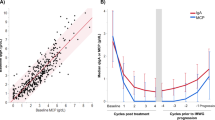
Serum FLC assays have proven useful markers of progressive disease, and may identify relapse earlier than traditional methods including urine [5, 31, 32]. In IIMM patients who relapse early after successful treatment, the short half-life of sFLCs offer a distinctive advantage over serum IFE for detecting progression, particularly in IgG MM patients [33]. Direct comparisons between serum FLC and 24 h urine also indicate that the former have greater sensitivity for detecting residual disease preceding clinical relapse [34] and for identifying light chain escape [35,36,37].
Serum FLCs for response assignment
Dispenzieri appraised serum and urine FLC measurements as markers of response, and demonstrated the lack of correlation between methods after just two treatment cycles [22]. The study also showed that early sFLC responses predicted for eventual overall response; however, there was no association with progression-free survival (PFS) and overall survival (OS). Dejoie also reported discrepancies between sFLC and 24 h urine assessment after three treatment cycles, but in this case sFLC measurements associated with both PFS and OS [27]. Fundamental differences between these studies were in patient selection and the induction protocol, which in Dispenzieri included all MM patients treated with obsolete therapy (VBMCP; vincristine, carmustine, melphalan, cyclophosphamide, and prednisone), whereas for Dejoie was restricted to LCMM patients undergoing induction with novel agents (VRD).
A limitation of the latter study was the lack of bone marrow data at the end of induction, which prevented the authors from determining how many patients with negative urine IFE were in CR. Since normalisation of urine at this stage did not associate with improved outcomes, it seems reasonable to hypothesise that 24 h urine measurements were not reflective of bone marrow plasma cell (BMPC) content, for BMPC < 5% post-induction with novel agents has been reported to associate with longer PFS and OS, at least in the transplant setting [38, 39]. By contrast, normalisation of sFLC parameters in the same study translated into superior PFS, OS and as predictors of immunophenotypic response, substantiating the argument that early responses based on sFLC changes are possibly more representative of tumour response.
Accurate monitoring shortly after initiation of treatment has become an ever growing clinical necessity in the era of novel drugs, with potential economic implications. Deeper responses during induction therapy with bortezomib-containing regimens are associated with improved outcomes in newly diagnosed patients [40, 41]. Conversely, failure to achieve early haematological response translates into inferior survival. Based on these distinct outcomes, a recent report advocated the inclusion of patient stratification in future MM trial design based on quality of response during induction therapy. The observation that poor responders may benefit from early dose-escalation is particularly relevant in countries with restricted funding for novel agents [42]. In this context, the better sensitivity of sFLC over 24 h urine measurements may offer more accurate monitoring conducive to cost-effective treatment decisions [43, 44].
The impact of replacing 24 h urine for serum FLC measurements has also been investigated at maximum response. In a cohort of 25 LCMM and 157 IIMM patients from the IFM2007 MM trial [23], responses based on serum methods (SPE + sFLC) demonstrated near-perfect agreement to standard SPE + uIFE assessment by IMWG guidelines. The main limitations of this and other comparative studies have been the lack of bone marrow samples to ascertain CR and the absence of results correlating response and clinical outcome. In an unpublished study including 450 IIMM patients from the IFM2009 trial, responses were assigned post-consolidation and post-maintenance therapy using IMWG response criteria; or modified criteria replacing 24 h urine for serum FLC assessment. As in previous reports, serum FLC measurements in this cohort were more sensitive than urines for identifying disease; however response assignment by both criteria, and PFS outcomes based on response, were comparable.
Conclusions
Although urine testing can provide useful information of underlying renal pathology due to glomerular or tubular dysfunction, and for screening patients with suspected AL amyloidosis [11, 45], there are many drawbacks to the use of 24 h urine BJP measurement for monitoring myeloma. Practical considerations include the difficulties to obtain complete 24 h collections from MM patients, who are often elderly and frail; the lack of standard protocols for the concentration of urine specimens; and poor compliance in the provision of a urine sample. However, renal physiology remains the most important issue concerning the use of urine for monitoring FLCs. Renal metabolism causes in most circumstances urinalysis to be an insensitive test for measuring monoclonal light chains, being primarily responsible for the lack of correlation with serum tests.
Replacing 24 h urine for sFLC measurements for monitoring IIMM patients results in equivalent response assignment, which in these patients relies mostly on M-protein changes as determined by SPE. This complements previous observations demonstrating clinical superiority of serum compared to urine assessment for monitoring LCMM patients. There is now cumulative and substantial evidence supporting the clinical and practical benefit of sFLC measurements for monitoring myeloma. We suggest the inclusion of sFLC to replace 24 h urine for the assignment of response in all MM patients, and propose modified response criteria modelled on those currently recommended by the IMWG (Table 1). Such criteria are already in place for monitoring oligosecretory patients, therefore our proposal would help to unify, and consequently simplify, current response criteria, making it pertinent to a greater proportion of patients.
References
Levinson SS, Keren DF. Free light chains of immunoglobulins: clinical laboratory analysis. Clin Chem. 1994;40:1869–78.
Siegel DS, McBride L, Bilotti E, Lendvai N, Gonsky J, Berges T, et al. Inaccuracies in 24-hour urine testing for monoclonal gammopathies. Lab Med. 2009;40:341–4.
Wochner RD, Strober W, Waldmann TA. The role of the kidney in the catabolism of Bence Jones proteins and immunoglobulin fragments. J Exp Med. 1967;126:207–21.
Maack T, Johnson V, Kau ST, Figueiredo J, Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979;16:251–70.
Alyanakian MA, Abbas A, Delarue R, Arnulf B, Aucouturier P. Free immunoglobulin light-chain serum levels in the follow-up of patients with monoclonal gammopathies: correlation with 24-hr urinary light-chain excretion. Am J Hematol. 2004;75:246–8.
Beetham R, Wassell J, Wallage MJ, Whiteway AJ, James JA. Can serum free light chains replace urine electrophoresis in the detection of monoclonal gammopathies? Ann Clin Biochem. 2007;44:516–22.
McTaggart MP, Lindsay J, Kearney EM. Replacing urine protein electrophoresis with serum free light chain analysis as a first-line test for detecting plasma cell disorders offers increased diagnostic accuracy and potential health benefit to patients. Am J Clin Pathol. 2013;140:890–7.
Holding S, Spradbery D, Hoole R, Wilmot R, Shields ML, Levoguer AM, et al. Use of serum free light chain analysis and urine protein electrophoresis for detection of monoclonal gammopathies. Clin Chem Lab Med. 2011;49:83–88.
Robson EJD, Taylor J, Beardsmore C, Basu S, Mead G, Lovatt T. Utility of serum free light chain analysis when screening for lymphoproliferative disorders. Lab Med. 2009;40:325–9.
Bradwell AR, Smith L, Drayson MT, Mead GP, Carr Smith HD. Serum free light chain measurements for identifying and monitoring patients with nonsecretory myeloma. Clin Chem Lab Med 2001;39:PO-F038a.
Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H, Hajek R, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215–24.
Rajkumar SV, Dimopolous MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548.
Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–23.
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–5.
Moreau P, Attal M, Facon T. Frontline therapy of multiple myeloma. Blood. 2015;125:3076–84.
Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum free light chains for monitoring multiple myeloma. Br J Haematol. 2004;126:348–54.
Bradwell AR, Carr-Smith HD, Mead GP, Harvey TC, Drayson MT. Serum test for assessment of patients with Bence Jones myeloma. Lancet. 2003;361:489–91.
Tate J, Caldwell G, Daly J, Gillis D, Jenkins M, Jovanovich S, et al. Recommendations for standardized reporting of protein electrophoresis in Australia and New Zealand. Ann Clin Biochem. 2012;49:242–56.
Abraham RS, Clark RJ, Bryant SC, Lymp JF, Larson T, Kyle RA, et al. Correlation of serum immunoglobulin free light chain quantification with urinary Bence Jones protein in light chain myeloma. Clin Chem. 2002;48:655–7.
Nowrousian MR, Brandhorst D, Sammet C, Kellert M, Daniels R, Schuett P, et al. Serum free light chain analysis and urine immunofixation electrophoresis in patients with multiple myeloma. Clin Cancer Res. 2005;11:8706–14.
Dispenzieri A, Zhang L, Katzmann JA, Snyder M, Blood E, DeGoey R, et al. Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008;111:4908–15.
Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101:356–62.
Corre J, Dejoie T, Caillon H, Attal M, Avet-Loiseau H, Moreau P. Serum free light chains should be the target of response evaluation in light chain multiple myeloma rather than urines: results from the IFM/DFCI 2009 trial. Blood. 2014;124:180a.
Boyle E, Brioli A, Leleu X, Morgan G, Pawlyn C, Davies F, et al. The value of serum free light chain monitoring compared to urinary Bence-Jones measurement in light chain only myeloma. Blood. 2013;122:1895a.
Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013;31:4529–35.
Dejoie T, Corre J, Caillon H, Hulin C, Perrot A, Caillot D, et al. Serum free light chains, not urine specimens, should be used to evaluate response in light-chain multiple myeloma. Blood. 2016;128:2941–8.
Lopez-Anglada Fernandez L, Cueto-Felgueroso C, Mateos MV, Rosinol L, Oriol A, Teruel AI, et al. Serum-free light-chains (sFLC) instead of urine protein electrophoresis (UPEP) for monitoring light-chain multiple myeloma (LCMM). Haematologica. 2017;102:E1278a.
Paiva B, Martinez-Lopez J, Vidriales MB, Mateos MV, Montalban MA, Fernandez-Redondo E, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29:1627–33.
Moustafa MA, Rajkumar SV, Dispenzieri A, Gertz MA, Lacy MQ, Buadi FK, et al. Utility of serum free light chain measurements in multiple myeloma patients not achieving complete response to therapy. Leukemia. 2015;29:2033–8.
Willenbacher E, Gasser S, Gastl G, Willenbacher W. Serum & urine free light chain analysis compared to conventional paraprotein measurements: usefulness for clinical decision making in real life haematology. Blood. 2009;114:2889a.
Mösbauer U, Ayuk F, Schieder H, Lioznov M, Zander AR, Kroger N. Monitoring serum free light chains in patients with multiple myeloma who achieved negative immunofixation after allogeneic stem cell transplantation. Haematologica. 2007;92:275–6.
Fuchida SI, Okano A, Hatsuse M, Murakami S, Haruyama H, Itoh S, et al. Serial measurement of free light chain detects poor response to therapy early in three patients with multiple myeloma who have measurable M-proteins. Int J Hematol. 2012;96:664–8.
Staderini M, Berardi M, Caldini A, Nozzoli C, Antonioli E, Bosi A. Role of serum free light chain vs bence jones measurement in light chain multiple myeloma (LCMM) at diagnosis, during treatment and follow-up for response evaluation and relapse detection. Haematologica. 2017;102:E1270a.
Caldini A, Nozzoli C, Terreni A, Staderini M, Berardi M, Biagioli T, et al. New patterns of relapse in multiple myeloma: a case of “light chain escape” in which FLC predicted relapse earlier than urine and serum immunofixation. Clin Chem Lab Med 2015;54:991–5.
Brioli A, Giles H, Pawlyn C, Campbell J, Kaiser M, Melchor L, et al. Serum free light chain evaluation as a marker for the impact of intra-clonal heterogeneity on the progression and treatment resistance in multiple myeloma. Blood. 2014;123:3414–9.
Kuhnemund A, Liebisch P, Bauchmuller K, Zur Hausen A, Veelken H, Wasch R, et al. ‘Light-chain escape-multiple myeloma’-an escape phenomenon from plateau phase: report of the largest patient series using LC-monitoring. J Cancer Res Clin Oncol. 2009;135:477–84.
Lee SE, Yoon JH, Shin SH, Eom KS, Kim YJ, Kim HJ, et al. Bone marrow plasma cell assessment before peripheral blood stem cell mobilization in patients with multiple myeloma undergoing autologous stem cell transplantation. Biomed Res Int. 2014;2014:982504.
Chakraborty R, Muchtar E, Kumar SK, Buadi FK, Dingli D, Dispenzieri A, et al. Impact of pre-transplant bone marrow plasma cell percentage on post-transplant response and survival in newly diagnosed multiple myeloma. Leuk Lymphoma 2017;58:308–15.
Harousseau JL, Palumbo A, Richardson PG, Schlag R, Dimopoulos MA, Shpilberg O, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood. 2010;116:3743–50.
Moreau P, Attal M, Pegourie B, Planche L, Hulin C, Facon T, et al. Achievement of VGPR to induction therapy is an important prognostic factor for longer PFS in the IFM 2005-01 trial. Blood. 2011;117:3041–4.
Chee A, Low MS, Vilcassim S, Gregory GP, Gilbertson M, Ratnasingam S, et al. Failure to achieve early disease response is associated with inferior survival in patients with newly diagnosed multiple myeloma. Br J Haematol 2018;182:739–41.
Fonseca R, Abouzaid S, Bonafede M, Cai Q, Parikh K, Cosler L, et al. Trends in overall survival and costs of multiple myeloma, 2000-2014. Leukemia. 2017;31:1915–21.
D’Souza A, Zhang MJ, Huang J, Fei M, Pasquini M, Hamadani M, et al. Trends in pre- and post-transplant therapies with first autologous hematopoietic cell transplantation among patients with multiple myeloma in the United States, 2004-2014. Leukemia. 2017;31:1998–2000.
Mikhael J. Pee no more? Urine light chains down the drain. Blood. 2016;128:2873–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dejoie, T., Corre, J., Caillon, H. et al. Responses in multiple myeloma should be assigned according to serum, not urine, free light chain measurements. Leukemia 33, 313–318 (2019). https://doi.org/10.1038/s41375-018-0339-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-018-0339-y