Abstract
Adrenomedullin (ADM), a member of the calcitonin family of peptides, is a potent vasodilator and was shown to have the ability to modulate bone metabolism. We have previously found a unique cell surface antigen (Kat1 antigen) expressed in rat osteoclasts, which is involved in the functional regulation of the calcitonin receptor (CTR). Cross-linking of cell surface Kat1 antigen with anti-Kat1 antigen monoclonal antibody (mAbKat1) stimulated osteoclast formation only under conditions suppressed by calcitonin. Here, we found that ADM provoked a significant stimulation in osteoclastogenesis only in the presence of calcitonin; a similar biological effect was seen with mAbKat1 in the bone marrow culture system. This stimulatory effect on osteoclastogenesis mediated by ADM was abolished by the addition of mAbKat1. 125I-labeled rat ADM (125I-ADM)-binding experiments involving micro-autoradiographic studies demonstrated that mononuclear precursors of osteoclasts abundantly expressed ADM receptors, and the specific binding of 125I-ADM was markedly inhibited by the addition of mAbKat1, suggesting a close relationship between the Kat1 antigen and the functional ADM receptors expressed on cells in the osteoclast lineage. ADM receptors were also detected in the osteoclast progenitor cells in the late mitotic phase, in which only one daughter cell of the dividing cell express ADM receptors, suggesting the semiconservative cell division of the osteoclast progenitors in the initiation of osteoclastogenesis. Messenger RNAs for the receptor activity-modifying-protein 1 (RAMP1) and calcitonin receptor-like receptor (CRLR) were expressed in cells in the osteoclast lineage; however, the expression of RAMP2 or RAMP3 was not detected in these cells. It is suggested that the Kat1 antigen is involved in the functional ADM receptor distinct from the general ADM receptor, consisting of CRLR and RAMP2 or RAMP3. Modulation of osteoclastogenesis through functional ADM receptors abundantly expressed on mononuclear osteoclast precursors is supposed to be important in the fine regulation of osteoclast differentiation in a specific osteotrophic hormonal condition with a high level of calcitonin in blood.
Similar content being viewed by others
Introduction
Adrenomedullin (ADM) is a member of the calcitonin family of peptides bearing the vasodilating activity on blood vessels [1, 2]. ADM acts on endothelial cells of the blood vessels through its specific receptors, the cell surface protein complex of the calcitonin receptor (CTR)-like receptor (CRLR) and receptor activity-modifying protein-2 (RAMP2) or receptor activity-modifying protein-3 (RAMP3), resulting in the production of local vasodilating factors to loosen the surrounding smooth muscle cells [3, 4]. ADM could also directly act on vascular smooth muscle cells, causing vasodilation. In sepsis, it is known that vascular leakage occurs through blockage of the endothelial cell barrier, which causes the severity of sepsis [5]. ADM is now showing promise as an effective therapeutic target for sepsis. ADM treatment contributes to the recovery of the leakage of endothelial cell barrier [6], in which administered ADM acts on endothelial cells to enforce cell–cell adhesion with adjacent endothelial cells to strengthen cell–cell adhesion through upregulating the expression of adhesion molecules.
Bone is a dynamic tissue that is frequently rebuilt by the continuous bone remodeling mediated by bone-resorbing osteoclasts and bone-forming osteoblasts. In the development of long bones, invasion of blood vessels is an essential step to induce calcification center in endochondral ossification. Recently, it has been revealed that blood vessels are composed of some subpopulation of endothelial cells [7]. In long bones, metaphyseal trabecular bone formation is supported by H-type blood vessels that are composed of CD31hi and Enmchhi endothelial cells, which induce the formation of osteoprogenitor cells in the metaphyseal areas [8, 9]. These findings suggest the regulatory roles of blood vessels on bone metabolism. Bone-resorbing osteoclasts are frequently observed in the metaphysis, especially on the surface of numerous bone trabecula.
Osteoclast differentiation is mainly mediated by the osteoclast-inducing cytokine, receptor activator NF-κb ligand (RANKL), and macrophage-colony-stimulating factor [10, 11]; however, osteoclast differentiation is influenced by several local growth factors and inflammatory cytokines. Recently, we have shown that osteoclasts formed under the stimulation with inflammatory cytokine interleukin-1β are related to the highly activated osteoclasts, especially the pathologically activated osteoclasts [12]. With respect to the interaction of osteoclasts with blood vessels, preosteoclast-derived platelet-derived growth factor-BB (PDGF-BB) induces angiogenesis during coupling with osteogenesis [13]. Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) are secreted by osteoclasts and induce blood vessel formation in the cortical bone [14, 15].
We have previously found a unique cell surface antigen distinguished from CTR, but modulates the function of CTR expressed on rat osteoclasts [16]. This antigen, designated as Kat1 antigen, is specifically expressed on the cell surface of osteoclasts and their precursors. Our previous studies have demonstrated that a high level of Kat1-Ag was expressed on the basolateral sides of actively resorbing osteoclasts, newly formed osteoclasts, and the mononuclear precursors of osteoclasts [17,18,19]. Calcitonin is thought to be effective in some bone diseases accompanying severe bone destruction, for example, Paget’s disease [2]. The European Medicine Agency suggests the use of salmon calcitonin for the short-term administration using the minimal effective dose of calcitonin in Paget’s disease [20]. Calcitonin has been utilized as the specific suppressor of osteoclastic bone resorption as osteoclasts possess abundant CTRs. In the case of calcitonin therapy, it has been confronted with a problem that the suppressive effects of calcitonin on bone resorption do not prolong due to the escape phenomenon [2]. The mechanism of this escape phenomenon remains to be ambiguous. We have previously shown that cross-linking of cell surface Kat1-Ag with anti-Kat1-Ag monoclonal antibody (mAbKat1) stimulated osteoclastogenesis only in the presence of calcitonin [21], suggesting involvement of Kat1-Ag in the incidence of calcitonin escape phenomenon. In this paper, as a result of an extensive search for cytokines bearing similar activity as mAbKat1, we found that ADM has an activity similar to that of mAbKat1, in which ADM stimulated osteoclastogenesis only in the presence of calcitonin. We focused on investigating functional ADM receptors expressed on cells in the osteoclast lineage, which is related to osteoclast-specific Kat1 antigen. We demonstrate the direct detection of ADM receptors on the cell surface of osteoclast precursors and the activity of ADM to modulate osteoclastogenesis only under a suppressive condition with calcitonin. We also demonstrate a close association of osteoclasts with the specific type of blood vessels in vivo.
Materials and methods
Materials
Sprague–Dawley (SD) rats were obtained from CLEA Japan Inc. (Tokyo, Japan). Glutaraldehyde was from Nakalai Tesque (Kyoto, Japan) and paraformaldehyde was from Merck (Darmstadt, Germany). ADM (rat) and calcitonin gene-related peptide (CGRP) (rat) were purchased from Bachem AG (Bubendorf, Switzerland) and peptide laboratory (Osaka, Japan). Salmon calcitonin was obtained from Sigma (St. Louis, MD). 125I-labeled ADM (rat) (74 TBq/mmol) was purchased from Amersham Pharmacia Biotech (Buckinghamshire, UK). NR-M2 autoradiographic emulsion was obtained from Konica (Kyoto, Japan). 1α,25-Dihydroxyvitamin D3 was purchased from Enzo Life Sciences (Farmingdale, NY). Sephadex G10 and tartrate-resistant acid phosphatase kits were obtained from Millipore-Sigma (Burlington, MA). Anti-endomucin antibody rat mAb (IgG2a) and anti-cathepsin K antibody (E-7) mouse mAb (IgG3k) were obtained from Santa Cruz (Dallas, TX).
Animal treatment
All experimental animals were carefully treated and maintained in accordance with the Guide for the Care and Use of Laboratory Animals in Kyushu University.
Immunohistochemical analysis
Rats with adjuvant-induced arthritis (AA rats) were prepared as described previously [19, 22,23,24]. After 3 weeks of adjuvant injection, AA rats were anesthetized with isoflurane and fixed by perfusion in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) from the left ventricle of the heart. Ankle joints involving surrounding bones were excised and fixed in 4% PFA in PBS at 4 °C for overnight. Tissue blocks were decalcified in 10% EDTA in PBS at 4 °C for 4 weeks. After trimming into small pieces, tissue blocks were immersed in sucrose series, followed by embedding in OCT compounds and frozen in liquid nitrogen, and stored in −80 °C before use. Immunostaining was performed as follows. Cryosections (10 μm thickness) were prepared using the cryostat (Thermo ScientificTMHM560). After complete drying, sections were rinsed in PBS three times (1 min each) to remove OCT compounds and immersed in 10 mM glycine-PBS two times (3 min each). After blocking in 5% chick albumin (Sigma, St. Louis, MO) for 1 h and in 10% goat serum (Vector Laboratories, Burlingame, CA) for a further 1 h at room temperature, primary antibodies (anti-endomucin antibody rat mAb:SC-48353, anti-cathepsin K antibody mouse mAb:SC-48353) were reacted with each section after 100-fold dilution in 1% bovine serum albumin/PBS (BSA/PBS), followed by incubation for 4 h at room temperature in a moisture chamber. After washing ten times with PBS, the secondary antibody mixture (Alexa FluorTM 488 goat anti-rat IgG H + L and Alexa FluorTM 568 goat anti-mouse IgG H + L) was added. After incubation at room temperature for 30 min, sections were rinsed in PBS and further treated with 4′,6-diamidine-2′-phenylindole dihydrochloride to stain the nuclei (Lonza, Basel, Switzerland, 50× dilution in PBS) for 1.5 min. After washing with PBS five times, sections were mounted using Aqua-Poly/Mount (Polysciences Inc. Warrington, PA). Each section was observed in laser scanning microscopy (Nikon C2si, Tokyo, Japan). In situ detection of Kat1 antigen was performed as described previously [17]. Briefly, mAbKat1 (100 μg) was injected intraperitoneally into newborn rats (SD rats). After 14 h, these rats were deeply anesthetized with isofluran and fixed by perfusion of 4% PFA/PBS from the left ventricle. Mandibula and maxilla were excised and further fixed in 4% PFA/PBS at 4 °C overnight. After decalcifying in 10% EDTA, tissue blocks were dehydrated in a series of ethanol solutions, then immersed in xylene, and finally embedded in paraffin. Paraffin sections of 4 μm thickness were deparaffinized in xylene and rehydrated using ethanol series. Localization of injected mAbKat1 (mouse IgM) was detected using anti-mouse IgM (μ-chain-specific) antibody conjugated with FITC.
Bone marrow cultures
For the formation of osteoclastic multinucleated cells (MNCs) and preosteoclast-like cells, bone marrow cells were obtained from the tibia and femur of SD rats and cultured as described previously [16, 19, 21,22,23,24]. For the formation of osteoclast-like MNCs, bone marrow cells were cultured (106 cells per well) in wells of 24-multiwell culture plates (Becton Dickinson Labware, Franklin Lakes, NJ) in α-minimum essential medium (α-MEM) supplemented with 15% FBS, 10% heat-treated ROS 17/2.8 cell-conditioned medium (htROSCM), and 10−8 M 1α,25-dihydroxyvitamin D3 in the presence of various concentrations of salmon calcitonin and rat ADM for 4 days, followed by tartrate-resistant acid phosphatase (TRAP) staining.
125I-ADM-binding studies and autoradiography
Bone marrow cells were cultured for 4 days in the presence of 10% (v/v) htROSCM, 10−8 M of 1α,25-dihydroxyvitamin D3, 20 ng/ml RANKL, and 50 ng/ml tumor necrosis factor-α. ADM receptors were detected by autoradiography using 125I-labeled rat ADM (125I-ADM). Cells were rinsed once with serum-free α-MEM containing 0.1% BSA at room temperature and incubated for 1 h at room temperature with 37 MBq/ml (2.92 ng/ml) 125I-ADM (Amersham Pharmacia Biotech, Little Chalfont, England; 74 MBq/mmol) in the absence or presence of excess (5 μg/ml) unlabeled ADM with or without 100 μg/ml of mAbKat1. Cells were rinsed with serum-free α-MEM containing 0.1% BSA three times. For measuring cell-bound radioactivity, these cells were immediately lysed in 0.5 N of NaOH, and then all dissolved materials were transferred into plastic tubes, followed by counting radioactivity using the γ-well counter. For micro-autoradiographic analysis, 125I-ADM-bound cells were fixed with PBS containing 2% formaldehyde and 2% glutaraldehyde for 20 min at room temperature. Cells were washed with PBS once and processed for TRAP staining only for 30 s to recognize TRAP-positive cells without affecting the detection of autoradiographic silver grains. After rinsing in deionized water, cells were dried up and the bottom of each well (culture surface) was cut out from the culture plates, dipped in NR-M2 emulsion (Konica Medical Co., Tokyo, Japan), and air-dried, followed by exposure for 19 days at 4 °C in light-shielding conditions. The autoradiographs were developed with Konicadol and fixed with Konifix (Konica Medical Co., Tokyo, Japan).
RT-PCR
Total RNAs were extracted from the following three cultures: (a) Whole bone marrow culture for forming osteoclasts as well as osteoblasts; (b) stromal cell-depleted bone marrow culture for forming cells in the osteoclast lineage; and (c) calvaria-derived primary osteoblasts. Total RNAs were extracted using Isogen (Nippon Gene, Toyama, Japan) and subjected to semiquantitative reverse transcription polymerase chain reaction (rtPCR) using RT-PCR kit (Takara Bio. Otsu, Japan) according to the manufacturer’s protocol. After synthesizing complementary DNA from the total RNA using oligo-dT primers, PCR reactions were performed using the following primers:
rat RAMP1 forward, 5′-CTCTGCTTGCCATGGCCCTC-3′,
rat RAMP1 reverse, 5′-CTCTGTGCGCTTGCTCCTCC-3′;
rat RAMP2 forward, 5′-CCAAGGCGTGATGGCTCCGC-3′,
rat RAMP2 reverse, 5′-AGAGGCGGAGCCTACGCCTG-3′;
rat RAMP3 forward, 5′-CACAGCGGCTGCACCTTCTC-3′,
rat RAMP3 reverse, 5′-CAGGCAGCAGGCTAGACAGC-3′;
rat CRLR forward, 5′-GGTACCACTACTTGGCATTG-3′,
rat CRLR reverse, 5′-GTGCACATCGCTGATTGTTG-3′.
PCR products were amplified using the following parameters, 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 1 min. PCR products were subjected to electrophoresis on 2% agarose gels and visualized by ethidium bromide staining with ultraviolet light illumination.
Preparation of primary osteoblasts
Primary osteoblasts were prepared from calvaria of newborn SD rats as described previously [25,26,27]. Briefly, collected calvaria was cut into pieces and exposed to five sequential enzymatic digestions using 0.1% collagenase type I and 0.2% dispase II (Wako Pure Chemicals, Tokyo, Japan) [25,26,27].
Statistical analysis
All data obtained from bone marrow cultures were analyzed using Student’s t test and post-analysis of variance test.
Results
Close association of osteoclasts with blood vessels
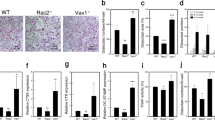
Figure 1a–c shows the localization of osteoclasts detected with osteoclast-specific mAbKat1 in mandibular tissues of newborn rats. Numerous osteoclasts stained with mAbKat1 were localized on the surface of trabecular bones. Blood vessels were frequently observed in the vicinity of osteoclasts, suggesting some modulatory roles of blood vessels in osteoclastogenesis and osteoclastic bone resorption. In the inflammatory bone tissues in AA rats, we have previously reported that a marked formation of osteophytes towards the lateral direction is frequently associated with severe bone destruction in the distal tibia as well as in the tarsus [22]. In bone destruction sites of the distal tibia, it was observed that osteoclasts were localized in contact with the endothelial cells expressing endomucin, and in the endothelial cell subtype composing H-type blood vessels related to bone formation (Fig. 1d). These data suggest a possible regulatory role of such bone-forming blood vessels in the pathological osteoclast formation in the inflammatory bone destruction sites.
a–c Localization of osteoclasts and blood vessels in normal bone tissues. Osteoclast-specific mAbKat1 was systemically injected into newborn rats to detect osteoclasts via bloodstream, followed by processing histological detection of osteoclasts as described in “Materials and methods.” Paraffin sections were prepared and further stained with anti-mouse IgM (μ-chain-specific) antibody conjugated with FITC to detect mAbKat1 (IgM) bound to osteoclasts. After observation under a fluorescence microscope, sections were further stained with HE. Figures show phase-contrast view (a), fluorescence microscopic observation (b), and HE staining (c) of the same section. B bone, BM bone marrow, BV blood vessel. Arrowheads: osteoclasts. Osteoclasts expressing Kat1 antigen are frequently observed in the vicinity of blood vessels in the bone marrow cavity. d Close association of endomucin-positive blood vessels with osteoclasts in bone destruction sites. Frozen sections of distal tibia of rats with adjuvant-induced arthritis were stained with anti-cathepsin K monoclonal antibody (mouse IgG) and anti-endomucin antibody (rabbit IgG), followed by detection with anti-mouse IgG conjugated with Alexa Fluor 568 and anti-rabbit IgG conjugated with Alexa Fluor 488 as described in “Materials and methods.” After staining the nuclei using DAPI, these sections were observed using the confocal laser scanning microscope. B bone, BM bone marrow, OC cathepsin K-positive osteoclasts. Arrow: endomucin-positive endothelial cell (End). Cross-section of endomucin-positive capillary was observed in close contact with cathepsin K-positive osteoclasts possibly performing bone destruction. Bars: 50 μm.
Stimulation of osteoclastogenesis by ADM in the presence of calcitonin
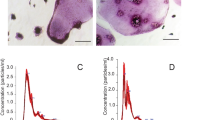
In the previous study, we have shown that cross-linking of cell surface Kat1 antigen specifically expressed on cells in the osteoclast lineage using mAbKat1 augmented osteocastogenesis only in the presence of calcitonin but not in the absence of calcitonin [21]. We have searched blood vessel-related cytokines and peptides bearing similar activity as mAbKat1 in osteoclastogenesis and found that ADM had an activity similar to mAbKat1. Figure 2 shows the dosage effect of ADM on the formation of osteoclastic MNCs in the presence of various concentrations of calcitonin. In the absence of calcitonin or in the presence of low concentrations (1 ng/ml) of calcitonin, no significant effect of ADM was observed (Fig. 2a, b). However, ADM significantly stimulated osteoclastic MNC formation in the presence of 10 ng/ml of calcitonin (Fig. 2c). The peak of the stimulation was ~1 ng/ml of ADM. In the presence of 100 ng/ml of calcitonin, significant suppression of osteoclastogenesis was observed in the absence of ADM; however, such inhibition was completely abolished by the addition of ADM (Figs. 2d and 3). Stimulatory effects of ADM on osteoclast formation in the presence of calcitonin were completely diminished by the addition of mAbKat1 as shown in Fig. 3. These data suggest that ADM-induced osteoclastogenesis was mediated through the cell surface molecule highly related to the osteoclast-specific Kat1 antigen.
Bone marrow cells were cultured to form osteoclastic MNCs in the presence of various concentrations of mAbKat1 with (b–d) or without (a) calcitonin as described in “Materials and methods.” Cells were stained for TRAP at day 4 of culture and the number of TRAP-positive MNCs was counted. a Formation of osteoclastic MNCs was not affected by any concentration of ADM Kat1, in the absence of calcitonin; b–d 1, 10, and 100 ng/ml calcitonin (CT). Significant stimulation of osteoclastic MNC formation by ADM was observed in the presence of 10–100 ng/ml of calcitonin. Data show the mean number of TRAP-positive MNCs ± SEM from quadruplicate cultures. These data represent a typical experiment from three independent experiments. *P < 0.05 and **P < 0.01.
Bone marrow cells were cultured to form osteoclastic MNCs in the presence of RANKL with or without calcitonin (CT) (100 ng/ml). These cells were treated with or without ADM (1 ng/ml) in the presence or absence of mAbKat1 (100 μg/ml). Data show the mean number of TRAP-positive MNCs ± SEM from quadruplicate cultures. These data represent a typical experiment from three independent experiments. Data were statistically analyzed as described in “Materials and methods.” Calcitonin inhibited osteoclast-like MNC formation; however, in the presence of ADM, the formation of osteoclast-like MNCs was markedly stimulated. This stimulation induced by ADM was completely suppressed by the addition of mAbKat1. *P < 0.05 if compared with the control culture containing no CT, ADM, and mAbKat1. #P < 0.05 if compared with each indicated pair.
Demonstration of ADM receptors in mononuclear precursors of osteoclasts
To investigate whether cells in the osteoclast lineage express specific receptors for ADM, we performed the binding experiments of 125I-ADM using bone marrow cultures for osteoclastogenesis. Figure 4 shows the binding of 125I-ADM to whole bone marrow cultures containing multinucleated osteoclast-like cells and mononuclear precursors of osteoclasts. Total binding of 125I-ADM (2.92 ng/ml) to these cells was markedly inhibited by the presence of an excess amount of unlabeled ADM (5 μg/ml, 1712-fold higher concentration in comparison to that of 125I-ADM), which shows the level of the nonspecific binding. The difference between the total binding and the nonspecific binding means the specific binding of 125I-ADM to the ADM receptors. These data clearly demonstrate the actual presence of the specific receptors for ADM in whole bone marrow cultures for forming osteoclasts. When mAbKat1 was added instead of unlabeled ADM, the level of the total 125I-ADM binding was also significantly suppressed, while the control antibody did not significantly inhibit 125I-ADM binding to these cells. As mAbKat1 specifically reacts with multinucleated osteoclasts as well as with mononuclear osteoclast precursors in rat bone marrow cultures for osteoclastogenesis [16, 18, 19], significant suppression in the total binding of 125I-ADM to these cells in the presence of osteoclast-specific mAbKat1 osteoclast cultures strongly suggest that mAbKat1 interfered with the specific binding of 125I-ADM to ADM receptors expressed on cells in the osteoclast lineage.
Bone marrow cells were cultured to form osteoclast-like MNCs in the presence of RANKL and 125I-ADM-binding experiments were performed as described in “Materials and methods.” The vertical line shows the radioactivity per culture. Marked inhibition of 125I-ADM (2.92 ng/ml) was observed in the presence of an excess amount (5000 ng/ml) of unlabeled (cold) ADM. The mAbKat1 also suppressed the binding of 125I-ADM to cells in the culture of osteoclast formation. These data demonstrate the actual presence of specific receptors for ADM in the culture of osteoclastogenesis, which are related to the Kat1 antigen. Data show the mean radioactivity ± SEM of 125I-ADM bound to cultured cells for osteoclastogenesis from quadruplicate cultures. Data represent a typical experiment from three independent experiments. ***P < 0.001 if compared to the total binding of 125I-ADM.
To know the type of cells that express specific receptors for ADM in the culture of osteoclastogenesis, micro-autoradiographic analysis was performed after binding of 125I-ADM to cultures for osteoclastogenesis. As shown in Fig. 5a–d, low levels of silver grains were detected on multinucleated osteoclasts (Fig. 5a), but quite a high level of silver grains was detected on mononuclear osteoclast precursors (Fig. 5b, asterisks). Few silver grains were observed in the presence of an excess amount of unlabeled ADM (5000 ng/ml) (1712-fold higher concentration in comparison to that of 125I-ADM) (Fig. 5c). These data clearly show the presence of ADM-specific receptors on cells in the osteoclast lineage, especially on the mononuclear osteoclast precursors. Interestingly, proliferating osteoclast progenitors in the late mitosis phase express ADM-specific receptors only in one daughter cell of the osteoclast progenitors in cell division (Fig. 5b, inset: arrowhead). The addition of mAbKat1 almost completely inhibited 125I-ADM binding to mononuclear cells as well as to MNCs in these cultures as shown in Fig. 5d, e. Control antibody did not affect the binding of 125I-ADM to mononuclear cells in these cultures (Fig. 5e). These data strongly suggest that functional ADM receptor, related to the Kat1 antigen, is highly expressed on mononuclear precursors of osteoclasts.
a–d Autoradiographic demonstration of the specific receptors for ADM on cells in the osteoclast lineage. Bone marrow cells were cultured for forming osteoclast-like cells in the presence of RANKL as described in “Materials and methods.” After 4 days of culture, 125I-ADM binding experiments were performed and cells were faintly stained for TRAP to detect osteoclast-like cells. a Multinucleated osteoclastic cells bearing silver grains in the cell margin. b Mononuclear cells bearing numerous silver grains (asterisks). Insert: high magnification view of the dividing osteoclast progenitor cell. Arrowheads: mitotic progenitor cells bearing numerous silver grains only in one daughter cell. c Nonspecific binding of 125I-ADM in the presence of an excess amount of unlabeled ADM. No silver grains were detected in cells in the culture for osteoclastogenesis. d Marked suppression of 125I-ADM binding in the presence of mAbKat1. No silver grains were observed in cells in the culture. Arrows: osteoclasts. Bars: 50 μm. e MAbKat1 inhibited specific binding of 125I-ADM to mononuclear precursors of osteoclasts. Bone marrow cells were cultured to form osteoclastic MNCs and processed for 125I-ADM binding as described in “Materials and methods.” The number of mononuclear cells bearing autoradiographic silver grains was counted. The mAbKat1 markedly suppressed the binding of 125I-ADM to mononuclear cells in culture, while the control antibody did not affect it at all. Data show the mean number of ADM receptor-positive mononuclear cells ± SEM from quadruplicate cultures. These data represent a typical experiment from three independent experiments. *P < 0.05 if compared to the total 125I-ADM binding. f Expression of CRLR and RAMP1 but not RAMP2 and RAMP3 in cells in the osteoclast lineage. Total RNA was prepared from each culture (lanes 1, 2, and 3 as shown below) and semiquantitative RT-PCR was performed to examine the expression of mRNAs for RAMP1, RAMP2, RAMP3, and CRLR. (Lane 1) Whole bone marrow culture cells involving stromal cells and cells in the osteoclast lineage. (Lane 2) Stromal cell-depleted bone marrow culture involving only cells in the osteoclast lineage. (Lane 3) Primary osteoblasts prepared from calvaria of newborn rats. Expression of CRLR and RAMP1 was detected in cells in the osteoclast lineage (lane 2).
We further examined the expression of RAMP1, RAMP2, RAMP3, and CRLR in cultures of osteoclastogenesis. Figure 5f shows semiquantitative rtPCR analysis of the expression of components of general ADM receptors. In whole bone marrow cultures containing osteoclasts and marrow stromal cells, the expression of messenger RNAs (mRNAs) for RAMP1, RAMP2, RAMP3, and CRLR was detected (Fig. 5f, lane 1). Calvaria-derived primary osteoblasts expressed RAMP1 and RAMP2 but not RAMP3 and CRLR (Fig. 5f, lane 3). In contrast, only RAMP1 and CRLR (Fig. 5f, lane 2) were detected in pure osteoclast cultures if we utilized cultures of stromal cell-depleted bone marrow cells. These data suggest that typical ADM receptors composed of RAMP2 (or RAMP3) and CRLR were not present in cells in the osteoclast lineage. As cells in the osteoclast lineage expressed RAMP1 and CRLR, we have examined if osteoclastogenesis was affected by CGRP, a ligand for RAMP1/CRLR complex. Although CGRP showed a limited inhibition of osteoclastogenesis in the presence of calcitonin, an increase in the osteoclast formation in the presence of calcitonin as observed in ADM treatment was not at all detected (Table 1). These data clearly demonstrate that CGRP signaling, possibly mediated through RAMP1 and CRLR, does not cause stimulation of osteoclastogenesis in the presence of calcitonin. It is suggested that CGRP signaling is not related to the ADM-mediated augment in osteoclastogenesis in the presence of calcitonin.
Discussion
In the current study, we have shown a close association of osteoclasts with blood vessels in normal bone. We further demonstrated a close relationship between endomucin-positive blood vessels and osteoclasts in inflammatory bone destruction sites. The endomucin-positive blood vessels have been reported to bear the potential activity to induce bone-forming osteoprogenitor cells [8, 9, 28]. As the marked lateral osteophyte formation with numerous bone spines is a characteristic of this bone destruction model [22], detection of endomucin-positive blood vessels could be related to such abnormal bone remodeling. These observations strongly suggest the presence of interaction between cells in the osteoclast lineage and blood vessels in normal bone tissues as well as in inflammatory bone tissues. It is known that endothelial cells secrete endothelin, which affects the osteoclastic function and osteoclast recruitment [29, 30]. We have previously reported that laminin, a major component of the extracellular matrix in the basement membrane of capillary vessels, suppresses osteoclastogenesis [31]. We have recently reported that Laminin 332 is also expressed in osteoblasts and suppresses osteoclastogenesis [32] in the normal bone marrow cavity. PDGF-BB, VEGF, and bFGF secreted by osteoclast precursors and osteoclasts are known to induce blood vessel formation, especially in the formation of Haversian canals and Folkman canals in the cortical bone [13,14,15].
ADM is a fundamental vasodilator systemically acting on the endothelial cells and blood smooth muscle cells to enlarge the diameter of blood vessels. ADM has been reported to have regulatory roles in bone metabolism. Cornish and co-workers reported that ADM acts on osteoblasts to augment bone formation [33, 34]. Liu et al. reported that ADM suppresses RANKL-induced osteoclastogenesis [35]; however, a detailed understanding of the target cells of ADM in cells in the osteoclast lineage are still ambiguous. In the current study, evaluated by the specific binding of 125I-ADM, we have shown quite a high expression of ADM receptors in the mononuclear osteoclast precursors. As 125I-ADM binding to these cells was specifically inhibited by the addition of osteoclast-specific mAbKat1, functional ADM receptors expressed on the cell surface of osteoclast precursors are highly related to the Kat1 antigen specifically expressed on cells in the osteoclast lineage. The level of the expression of ADM receptors was dramatically suppressed in multinuclear osteoclasts. Interestingly, the ADM receptors were often detected only in one daughter cell of the postmitotic dividing osteoclast progenitor cells. We have previously found that functional CTRs as well as the Kat1 antigen were expressed in one daughter cell of osteoclast progenitor cells in cell division [18]. These data strongly suggest that functional ADM receptors highly related to Kat1 antigen initiate to be expressed during the asymmetric cell division of the osteoclast progenitors. In other words, osteoclast differentiation is initiated from the postmitotic stage of the cell cycle of osteoclast progenitor cells (Fig. 6a). In the cell division of the stem cells, it has been reported that the distal daughter cell away from niche having incorporated old mitochondria initiates differentiation, while the proximal daughter cell bound to niche keeps stem cell properties [36]. Inaba et al. [37] reported that daughter cells connected via tunneling nanotubes with niche cells stay as the stem cell, while the distal daughter cell differentiates. It could be possible to target such asymmetric cell division of the osteoclast progenitors to modulate bone metabolism.
a Expression of functional ADM receptors in cells in the osteoclast lineage. ADM receptors initiate to be expressed in a high level on one daughter cell of the late mitotic osteoclast progenitor and continue to be expressed in mononuclear osteoclast precursors. The other daughter cell expressing no ADM receptors would remain as the osteoclast progenitor cell. In multinucleated osteoclasts, the expression level of the ADM receptor is markedly reduced in comparison to that of the mononuclear osteoclast precursors. b Possible involvement of Kat1 antigen in functional ADM receptors in cells in the osteoclast lineage. Cells in the osteoclast lineage express calcitonin receptor (CTR), CRLR, and RAMP1 in rat bone marrow culture for osteoclastogenesis. In mononuclear osteoclast precursors, Kat1 antigen could associate with CRLR, which forms specific receptors for ADM. In multinucleated osteoclasts, the Kat1 antigen may dissociate from CRLR to associate with CTR, which could contribute to the increase in the affinity of CTR to its ligand calcitonin [2, 16].
In this manuscript, we have shown that ADM is involved in the regulation of osteoclastogenesis under conditions suppressed by calcitonin. Calcitonin is the potent inhibitor of osteoclastic bone resorption and is utilized in several metabolic bone diseases, although worldwide the use of calcitonin is declining because of the appearance of more effective medicines [2]. In the clinical trial of calcitonin, osteoclasts are known to become insensitive to calcitonin and such osteoclasts cannot be regulated by this hormone. The mechanism of this clinically identified phenomenon, the so-called “escape phenomenon,” is thought to be attributed to the suppression of the expression of CTRs at the transcriptional level [38]; however, an alternative mechanism is supposed to be involved in the regulatory process of osteoclast formation. Studies using irradiated animals demonstrated that the new formation of osteoclasts replaced osteoclasts in which CTRs had been downregulated [39, 40]. The current study showed that the formation of osteoclast-like cells was significantly stimulated by ADM in the presence of calcitonin, and suppression of osteoclastogenesis mediated by 100 ng/ml of calcitonin was completely abolished by the addition of ADM. Such modulation caused by ADM could be involved in the incidence of the “calcitonin escape.”
In the current study, we have clarified that ADM is involved in the modulation of osteoclastogenesis under conditions suppressed by calcitonin. Such modulation of osteoclastogenesis mediated by ADM was abolished by the addition of mAbKat1. These data suggest that ADM induces the modulation of osteoclastogenesis through cell surface molecule Kat1 antigen specifically expressed in cells in the osteoclast lineage. Micro-autoradiographic studies showed that specific ADM receptors were abundantly expressed in mononuclear osteoclast precursors rather than in multinucleated osteoclasts. In contrast, the Kat1 antigen is highly expressed both on multinucleated osteoclasts and on mononuclear osteoclast precursors [16, 18, 19].
The family of membrane molecule RAMPs provides the functional receptors for ADM if RAMP2 or RAMP3 is associated with CRLR and alternatively provides the functional receptors for CGRP when RAMP1 is coupled with CRLR. In the current study, functional ADM receptors were abundantly expressed in mononuclear osteoclast precursors. Before performing the detection of mRNA, we have expected that RAMP2 (or RAMP3) and CRLR mRNA are expressed in osteoclast lineage, which could provide functional ADM receptors. However, semiquantitative rtPCR analysis showed that only RAMP1 and CRLR mRNAs were expressed in cells in the osteoclast lineage when stromal cell-deprived bone marrow culture was examined. In other words, we failed in detecting RAMP2 and RAMP3 in cells in the osteoclast lineage. Therefore, functional ADM receptors expressed on osteoclast precursors could not be the typical ADM receptors CRLR/RAMP2 or CRLR/RAMP3. Kat1 antigen could function like RAMP2 or RAMP3 to associate with CRLR, which results in providing the functional ADM receptor in osteoclast precursors. As the abundant functional ADM receptors were detected mainly on mononuclear osteoclast precursors but not in multinuclear osteoclasts, the frequency of association of Kat1 antigen with CRLR could be decreased during the multinucleation step, which could result in suppressing the number of the functional ADM receptors in multinucleated osteoclasts (Fig. 6b). In osteoclasts, cross-linking of cell surface Kat1 antigen causes a significant increase in the affinity of CTR [16]. A major coupling partner of the Kat1 antigen is supposed to be the CTR in multinucleated osteoclasts. In contrast, CRLR may associate with the Kat1 antigen instead of RAMP2 or RAMP3, which could give rise to a novel type of ADM receptors in the mononuclear osteoclast precursors (Fig. 6b). Specific regulation of ADM receptors expressed on mononuclear osteoclast precursors could provide one alternative way to modulate bone remodeling in the normal regulatory conditions of osteotrophic hormones as well as in the pathological bone destruction.
References
Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–60.
Xie J, Guo J, Kanwal Z, Wu M, Lv X, Ibrahim NA, et al. Calcitonin and bone physiology: in vitro, in vivo, and clinical investigations. Int J Endocrinol. 2020;2020:3236828.
McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Tompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–39.
Fischer JP, Els-Heindl S, Beck-Sickinger A. Adrenomedullin—current perspective on a peptide hormone with significant therapeutic potential. Peptides. 2020;131:170347.
Geven C, Kox M, Pickkers P. Adrenomedullin and adrenomedullin-targeted therapy as treatment strategies relevant for sepsis. Front Immunol. 2018;9:292
Hippenstiel S, Witzenrath M, Schmeck B, Hocke A, Krisp M, Krull M, et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res. 2002;91:618–25.
Langen UH, Pitulescu ME, Kim JM, Enriquez-Gasca R, Sivaraj KK, Kusambe AP, et al. Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat Cell Biol. 2017;19:189–201.
Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–80.
Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–28.
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–602.
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76.
Shiratori T, Kyumoto-Nakamura Y, Kukita A, Uehara N, Zhang JQ, Koda K, et al. IL-1beta Induces pathologically activated osteoclasts bearing extremely high levels of resorbing activity: a possible pathological subpopulation of osteoclasts, accompanied by suppressed expression of kindlin-3 and talin-1. J Immunol. 2018;200:218–28.
Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. 2014;20:1270–78.
Cappariello A, Maurizi A, Veeriah V, Teti A. The Great Beauty of the osteoclast. Arch Biochem Biophys. 2014;558:70–78.
Charles JF, Aliprantis AO. Osteoclasts: more than ‘bone eaters’. Trends Mol Med. 2014;20:449–59.
Kukita T, Kukita A, Nagata K, Maeda H, Kurisu K, Watanabe T, et al. Novel cell-surface Ag expressed on rat osteoclasts regulating the function of the calcitonin receptor. J Immunol. 1994;153:5265–73.
Kukita T, Kukita A, Xu L, Maeda H, Iijima T. Successful detection of active osteoclasts in situ by systemic administration of an osteoclast-specific monoclonal antibody. Calcif Tissue Int. 1998;63:148–53.
Kukita T, Kukita A, Xu L, Watanabe T, Iijima T. Kat1-antigen–a reliable immunological marker for identifying osteoclast precursors of rats: detection of subpopulations among precursors and initiation of osteoclastogenesis. Histochem Cell Biol. 2001;115:215–22.
Matsubara R, Kukita T, Ichigi Y, Takigawa I, Qu PF, Funakubo N, et al. Characterization and identification of subpopulations of mononuclear preosteoclasts induced by TNF-alpha in combination with TGF-beta in rats. PLoS ONE. 2012;7:e47930.
Langston AL, Ralston SH. Management of Paget’s disease of bone. Rheumatology 2004;43:955–59.
Kukita T, Kukita A, Watanabe T, Iijima T. Osteoclast differentiation antigen, distinct from receptor activator of nuclear factor kappa B, is involved in osteoclastogenesis under calcitonin-regulated conditions. J Endocrinol. 2001;170:175–83.
Kuratani T, Nagata K, Kukita T, Hotokebuchi T, Nakasima A, Iijima T, et al. Induction of abundant osteoclast-like multinucleated giant cells in adjuvant arthritic rats with accompanying disordered high bone turnover. Histol Histopathol. 1998;13:751–59.
Li YJ, Kukita A, Teramachi J, Nagata K, Wu Z, Akamine A, et al. A possible suppressive role of galectin-3 in upregulated osteoclastogenesis accompanying adjuvant-induced arthritis in rats. Lab Invest. 2009;89:26–37.
Moriyama K, Kukita A, Li YJ, Uehara N, Zhang JQ, Takahashi I, et al. Regulation of osteoclastogenesis through Tim-3: possible involvement of the Tim-3/galectin-9 system in the modulation of inflammatory bone destruction. Lab Invest. 2014;94:1200–11.
Takahashi N, Yamana H, Yoshiki S, Roodman GD, Mundy GR, Jones SJ, et al. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988;122:1373–82.
Kukita A, Kukita T, Shin JH, Kohashi O. Induction of mononuclear precursor cells with osteoclastic phenotypes in a rat bone marrow culture system depleted of stromal cells. Biochem Biophys Res Commun. 1993;196:1383–89.
Badawy T, Kyumoto-Nakamura Y, Uehara N, Zhang J, Sonoda S, Hiura H, et al. Osteoblast lineage-specific cell-surface antigen (A7) regulates osteoclast recruitment and calcification during bone remodeling. Lab Invest. 2019;99:866–84.
Romeo SG, Alawi KM, Rodrigues J, Singh A, Kusumbe AP, Ramasamy SK. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat Cell Biol. 2019;21:430–41.
Zaidi M, Alam AS, Bax BE, Shankar VS, Bax CMR, Gill JS, et al. Role of the endothelial cell in osteoclast control: new perspectives. Bone. 1993;14:97–102.
Ibrahimi Disha S, Furlani B, Drevensek G, Plut A, Yanagisawa M, Hudoklin S, et al. The role of endothelin B receptor in bone modelling during orthodontic tooth movement: a study on ETB knockout rats. Sci Rep. 2020;10:14226.
Kukita T, Hata K, Kukita A, Iijima T. Laminin, a major basement membrane component of the blood vessel, as a negative regulator of osteoclastogenesis. Calcif Tissue Int. 1998;63:140–42.
Uehara N, Kukita A, Kyumoto-Nakamura Y, Yamaza T, Yasuda H, Kukita T. Osteoblast-derived Laminin-332 is a novel negative regulator of osteoclastogenesis in bone microenvironments. Lab Invest. 2017;97:1235–44.
Naot D, Callon KE, Grey A, Cooper JS, Reid IR, Cornish J. A potential role for adrenomedullin as a local regulator of bone growth. Endocrinology. 2001;142:1849–57.
Cornish J, Naot D, Reid IR. Adrenomedullin—a regulator of bone formation. Regul Pept. 2003;112:79–86.
Liu Y, Zuo G, Meng X, Gao X, Zhang L, Tang P. Adrenomedullin inhibits osteoclast differentiation through the suppression of receptor activator of nuclear factor-kappaB ligand-induced nuclear factor-kappaB activation in glucocorticoid-induced osteoporosis. Exp Ther Med. 2017;14:4009–16.
Katajisto P, Dohla J, Chaffer CL, Pentinmikko N, Marjanovie N, Iqbal S, et al. Stem cells. Asymmetric apportioning of aged mitochondria between daughter cells is required for stemness. Science. 2015;348:340–43.
Inaba M, Buszczak M, Yamashita YM. Nanotubes mediate niche-stem-cell signalling in the Drosophila testis. Nature. 2015;523:329–32.
Takahashi S, Goldring S, Katz M, Hilsenbeck S, Williams R, Roodman GD. Downregulation of calcitonin receptor mRNA expression by calcitonin during human osteoclast-like cell differentiation. J Clin Invest. 1995;95:167–71.
Krieger NS, Feldman RS, Tashjian AH. Parathyroid hormone and calcitonin interactions in bone: irradiation-induced inhibition of escape in vitro. Calcif Tissue Int. 1982;34:197–203.
Nakamura T, Toyofuku F, Kanda S. Whole-body irradiation inhibits the escape phenomenon of osteoclasts in bones of calcitonin-treated rats. Calcif Tissue Int. 1985;37:42–45.
Author information
Authors and Affiliations
Contributions
TK and AK performed the study concept and design. TK, HH, TY, IT, and AK performed the development of methodology and writing, review, and revision of the paper. TK, HH, and AK provided acquisition of data and statistical analysis. JG, JZ, YK-N, NU, SM, and SS provided technical and material support. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Funding
This work was supported in part by a Grant for Scientific Research from the Japanese Ministry of Education, Science and Culture (Project 09671858, 15K15677, and 18K09506).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kukita, T., Hiura, H., Gu, JY. et al. Modulation of osteoclastogenesis through adrenomedullin receptors on osteoclast precursors: initiation of differentiation by asymmetric cell division. Lab Invest 101, 1449–1457 (2021). https://doi.org/10.1038/s41374-021-00633-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-021-00633-2