Abstract
Huntington’s disease (HD) is an autosomal dominant disorder caused by a trinucleotide expansion in the huntingtin gene. Recently, a new role for tau has been implicated in the pathogenesis of HD, whereas others have argued that postmortem tau pathology findings are attributable to concurrent Alzheimer’s disease pathology. The frequency of other well-defined common age-related tau pathologies in HD has not been examined in detail. In this single center, retrospective analysis, we screened seven cases of Huntington’s disease (5 females, 2 males, age at death: 47–73 years) for neuronal and glial tau pathology using phospho-tau immunohistochemistry. All seven cases showed presence of neuronal tau pathology. Five cases met diagnostic criteria for primary age-related tauopathy (PART), with three cases classified as definite PART and two cases as possible PART, all with a Braak stage of I. One case was diagnosed with low level of Alzheimer’s disease neuropathologic change. In the youngest case, rare perivascular aggregates of tau-positive neurons, astrocytes and processes were identified at sulcal depths, meeting current neuropathological criteria for stage 1 chronic traumatic encephalopathy (CTE). Although the patient had no history of playing contact sports, he experienced several falls, but no definitive concussions during his disease course. Three of the PART cases and the CTE-like case showed additional evidence of aging-related tau astrogliopathy. None of the cases showed significant tau pathology in the striatum. In conclusion, while we found evidence for tau hyperphosphorylation and aggregation in all seven of our HD cases, the tau pathology was readily classifiable into known diagnostic entities and most likely represents non-specific age- or perhaps trauma-related changes. As the tau pathology was very mild in all cases and not unexpected for a population of this age range, it does not appear that the underlying HD may have promoted or accelerated tau accumulation.
Similar content being viewed by others
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder caused by an abnormal CAG repeat expansion in exon 1 of the huntingtin gene [1]. Clinically, HD presents with progressive motor, cognitive, and psychiatric symptoms [2,3,4]. Neuropathologically, HD is characterized by progressive atrophy of the striatum, which forms the basis for the 5-tiered Vonsattel grading scheme [5]. At time of autopsy, most HD brains exhibit markedly reduced brain weights, which is a reflection of more widespread brain involvement with volume loss of cerebral cortex, white matter and limbic regions [4, 6]. Microscopically, neuronal loss in the striatum is accompanied by reactive astrogliosis and intranuclear, cytoplasmic and neuritic inclusions at varying densities, which can be detected in both striatum and cerebral cortex by immunohistochemistry using antibodies targeted at huntingtin, polyglutamine, or ubiquitin [7, 8]. In addition, fused in sarcoma (FUS), an RNA binding protein, was identified as a major component of intranuclear aggregates in polyglutamine diseases including HD [9], and showed greater sensitivity in detecting them compared to ubiquitin or p62 [10]. FUS-positive cellular inclusions are the histopathologic hallmark of rare forms of familial ALS [11, 12] and several rare subtypes of frontotemporal lobar degeneration, collectively termed FTLD-FUS [13,14,15], but are not observed in other neurodegenerative conditions with the exception of polyglutamine diseases [16].
Many other proteinopathies have been identified in HD brains [17,18,19,20], including alpha-synuclein and transactive response DNA-binding protein (TDP-43). Most attention, however, has been recently paid to the presence of co-existing tau pathologies. Two case series have identified neuronal tau pathology with or without associated beta-amyloid plaques in large proportions of HD cases, with higher frequency and severity in elderly subjects, meeting formal criteria for Alzheimer’s disease in a subset of cases [21, 22]. Whereas striatal tau deposition was not discussed in one of these studies [21], Jellinger et al. noted that no subcortical neurofibrillary tangles or neuropil threads were seen in any of their adult HD brains [22]. This is in contrast to two recent studies, describing a wide range of neuronal and glial tau deposits in the striatum of HD brains and an unusual form of rod-like tau deposits (TNR) spanning the neuronal nuclear space [23, 24]. These TNRs were thought to represent neuronal nuclear indentations filled with tau protein and were detected at much higher frequency in the cortex and striatum of HD compared to control brains. Furthermore, biochemical analyses of striatal tissue demonstrated abnormal tau phosphorylation in advanced disease stages and aberrant tau splicing with increased exon 10 inclusion, leading to higher 4R/3T tau isoform ratios in HD compared to control striatum [19, 23]. Consistent with the human findings, in vitro studies and rodent HD models showed evidence of interaction between mutant huntingtin and tau resulting in tau hyperphosphorylation [25]. Based on these findings, it has been postulated that HD may represent a new member of the family of tauopathies [26].
Tauopathies are a heterogeneous group of neurodegenerative diseases, defined by the presence of neuronal and/or glial tau inclusions with distinct morphological patterns, distribution, and isoform composition [27]. In recent years, tau pathologies have undergone re-classification, with age-related neuronal and astroglial tau aggregation now recognized as distinct entities, termed primary age-related tauopathy (PART) [28] and aging-related tau astrogliopathy (ARTAG) [29], respectively. PART describes the common presence of neurofibrillary tangle pathology in the brains of elderly individuals, mostly restricted to structures of the medial temporal lobe, and without accompanying amyloid deposition (definitive PART) or only minimal diffuse but no neuritic amyloid plaques (possible PART) [28]. ARTAG refers to the accumulation of hyperphosphorylated tau in astrocytes of the aging brain. The tau-positive astrocytes of ARTAG are defined by thorn-shaped or granular/fuzzy morphologies and are found in characteristic distribution patterns [29,30,31]. These pathologies can be found concurrently with many other neurodegenerative disease processes, but their presence in HD brains has not been evaluated so far. We present a series of seven HD cases, which were studied in detail for presence and distribution of phospho-Tau (pTau) aggregation and common age- or trauma-related tau pathologies.
Materials and methods
Patients
Seven cases of HD were identified in a database search of the Neurodegenerative Brain Bank at the University of Pittsburgh. The cases were collected between 1998 and 2017 with consent by next-of-kin and approval by the Committee for Oversight of Research and Clinical Training Involving Decedents (CORID). All cases carried a clinical diagnosis of HD which was confirmed by pathognomonic findings on postmortem examination. Information about CAG repeat expansion within the huntingtin gene was available in only two cases. Medical records were reviewed for information about disease course and symptoms. For one subject, clinical notes were available from the University of Pittsburgh Alzheimer’s disease research center (ADRC).
Neuropathology methods
After 1–2 weeks of immersion fixation in 10% neutral buffered formalin, all brains underwent standard diagnostic neuropathological examination. Coronal slices were reviewed grossly for severity of striatal atrophy and rated following the Vonsattel grading system [5]. Sections for microscopic examination were sampled from multiple anatomic regions using a standardized approach to include cerebral cortex from all four lobes, basal ganglia at multiple coronal levels, thalamus, hippocampus, amygdala, brainstem, and cerebellum. Five micrometers thick paraffin sections were stained for hematoxylin and eosin (H&E). Select cortical sections were stained with a modified Bielschowsky silver stain to screen for neuritic plaque pathology [32, 33]. Immunohistochemical stains for beta-amyloid, phospho-tau (pTau), alpha-synuclein and phospho-TDP were performed on select sections to screen for Alzheimer’s disease neuropathologic changes and other neurodegenerative pathologies as recommended by the 2012 National Institutes of Aging/Alzheimer’s Association guidelines [33, 34]. P-Tau immunostains were performed on all available sections. Additional phosphorylation-, conformation-, and isoform-dependent and independent tau antibodies were used on striatal sections. For cases that were banked before 2012, additional stains were performed as needed in order to re-classify these cases according to current guidelines. All cases were screened for the presence of intranuclear inclusions using antibodies against polyglutamine, p62 and fused in sarcoma (FUS).
Immunohistochemical staining was performed with the following pretreatment protocols, antibody dilutions, and sources: beta-amyloid (NAB228, 1:4000, formic acid; Cell Signaling, Danvers, MA), alpha-synuclein (LB509, 1:300, protease XXIV; Invitrogen, Thermo Fisher, Waltham, MA), tau (polyclonal, 1:200, no antigen retrieval; Dako Agilent, Santa Clara, CA), pTau ser396/ser404 (PHF1, 1:1000, citrate; kindly provided by Peter Davies, The Feinstein Institute for Medical Research, NY), pTau ser202 (CP13, 1:1000, citrate; kindly provided by Peter Davies), 3R tau isoform (RD3, 1:1000, citrate, Millipore, Burlington, MA), 4R tau isoform (ET3, 1:100, citrate, kindly provided by Peter Davies), oligomeric tau (T22, 1:1000, citrate, Millipore), phospho-TDP (1D3, 1:500, citrate; kindly provided by Manuela Neumann, Helmholtz Zentrum, München, Germany), polyglutamine (1C2, 1:500, citrate; Millipore), p62 (3/P62, 1:1000, citrate; BD Biosciences, San Jose, CA), FUS (HPA008784, 1:400, citrate; Sigma-Aldrich, St. Louis, MO) and splicing factor proline- and glutamine-rich (SFPQ; polyclonal, ab38148, 1:2000, citrate, Abcam, Cambridge, MA). Immunohistochemical signal was visualized using Vectastain Elite ABC HRP kit (Vector, Burlingame, CA) following the manufacturer’s instructions and NovaRED peroxidase substrate as chromogen (Vector). Slides were counterstained with Mayer’s hematoxylin (Sigma-Aldrich). To investigate a possible relationship between HD-associated intranuclear inclusions and SFPQ, double-labeling immunofluorescence studies were performed using Alexa Fluor 488 and Cy3-labeled secondary antibodies (both 1:200, Jackson ImmunoResearch, West Grove, PA) and DAPI nuclear counterstain (1:1000, Thermo Scientific, Waltham, MA).
Evaluation of tau pathology
All available sections from all seven cases were screened for the presence of PHF1-positive pTau aggregates in neurons and glial cells. If present, regional distribution, semiquantitative density (mild, moderate, and severe) and morphology were documented. Based on these features, tau pathology was classified according to current diagnostic guidelines for PART, ARTAG, AD, and other tauopathies as appropriate [27,28,29, 33,34,35].
Results
There were 5 females and 2 males in our study, ranging in age at death from 47 to 73 years. At least partial clinical information about neuropsychiatric manifestations was available on all of the subjects and is listed in Table 1.
Neuropathological findings are summarized in Table 2. With the exception of the youngest subject, all cases demonstrated reduced brain weights, with the majority weighing near or below 1000 g. All cases exhibited marked gross atrophy of the neostriatum, associated with severe neuronal loss and gliosis in these regions. Using the Vonsattel grading system, all cases met criteria for grades 3 or 4. Diagnosis was supported by the presence of characteristic intranuclear polyglutamine inclusions in striatal and neocortical neurons. The inclusions were also reactive for p62 and FUS (Fig. 1). FUS nuclear immunoreactivity was weaker in some but not all neurons bearing intranuclear FUS aggregates.
Screening of all available tissue sections using pTau immunohistochemistry revealed the presence of neuronal tau pathology in all cases. In six of the seven cases, the neuronal tau pathology consisted of a few neurofibrillary tangles and neuropil threads, which were restricted to the mesial temporal lobe (Fig. 2a). No neocortical tau pathology was present in any of these six cases, consistent with Braak stages of I or II. Three of the cases showed no evidence of beta-amyloid deposition and met criteria for definite PART [28]. Two cases had a few diffuse amyloid deposits in the neocortex, but no neuritic plaques, and were classified as possible PART. Only one case showed sparse neuritic plaques and subcortical amyloid deposition, meeting criteria for low-level Alzheimer’s disease neuropathologic change (A2, B1, C1) following the 2012 NIA-AA criteria for the neuropathologic assessment of AD [33, 34].
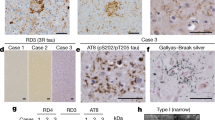
a Representative image of age-associated neuronal tau pathology, consisting of neurofibrillary tangles and neuropil threads in the entorhinal cortex. b–e Representative images of age-associated astrocytic tau pathologies, depicting examples of perivascular white matter b, subpial c, and subependymal d thorn-shaped astrocytes and rare granular/fuzzy cortical astrocytes e. f–i CTE-like pathology in case 1, consisting of perivascular aggregates at a sulcal depth f associated with thorn-shaped astrocytes in the overlying subpial zone g. H&E image of hemosiderin-containing glial scar in superficial entorhinal cortex h associated with thorn-shaped astrocytes in the overlying subpial zone i. All images except H are labeled with pTau antibody PHF1
The neuronal tau pathology in case 1, the youngest subject in our case series, followed a different distribution. Sections of midfrontal, precentral and insular cortex revealed perivascular aggregates of neurofibrillary tangles, neuropil threads, a few dot-like structures, and rare glial inclusions (Fig. 2f). Some but not all of these aggregates were located at the depth of cortical sulci. Most other neocortical sections contained a few scattered neurofibrillary tangles and neuropil threads without forming distinct aggregates. In the mesial temporal lobe, moderate neuronal tau pathology was seen in amygdala and CA1 sector of the anterior hippocampus, whereas only sparse tangles and threads were present in posterior hippocampus and entorhinal cortex. Given the presence of characteristic perivascular pTau aggregates, the findings were consistent with a neuropathological diagnosis of stage 1 chronic traumatic encephalopathy (CTE) [35, 36]. In retrospective interviews with the patient’s family, no history of contact sports participation was revealed. Although it was noted that he experienced several falls during his disease course, the family did not recollect any clinically symptomatic concussion events. No CTE-like changes were observed in any of the other cases.
In addition to neuronal tau pathology, we also identified pTau aggregation in astrocytes in four of the cases. In two of the PART cases, the glial tau pathology consisted predominantly of thorn-shaped astrocytes and was found in perivascular white matter, subpial and subependymal distributions in the anterior mesial temporal lobe and basal forebrain (Fig. 2b–d). In a third PART case, there was only minimal glial tau pathology in amygdala and frontal cortex, morphologically consistent with granular/fuzzy astrocytes (Fig. 2e). The morphology and distribution of the pTau positive astrocytes were characteristic of findings seen in aging-related tau astrogliopathy (ARTAG) [29].
Perivascular clusters of thorn-shaped astrocytes were also detected in the CTE case in the white matter of the anterior mesial temporal lobe and region of the nucleus basalis, which were considered to represent ARTAG. In addition, thorn-shaped astrocytes were found focally in a subpial location in the insular cortex (Fig. 2g), at the depth of a sulcus with an underlying perivascular neuronal tau aggregate, and thought to be CTE-related [31, 35]. Another small cluster of subpial thorn-shaped astrocytes was noted focally at the surface of the anterior entorhinal cortex, overlying a remote microscopic glial scar with hemosiderin deposition (Fig. 2h, i).
A wide range of phosphorylation-, isoform-, and conformation-dependent and independent tau antibodies was employed to screen for striatal tau pathology but only minimal changes were identified (Fig. 3). In five cases, very rare threads were seen and one of these cases also contained a very rare pre-tangle and thorn-shaped astrocyte. The remaining two cases were negative. No rod-like or other tau nuclear inclusions were identified in any of our cases.
Minimal tau pathology is present in the striatum. a–c Rare neuropil threads, d a very rare pre-tangle and e a very rare thorn-shaped astrocyte are seen. No nuclear rods are identified in residual striatal neurons f. Images a and e are labeled with pTau antibody PHF1, images b and d with pTau antibody CP13 and images c and e with phosphorylation-independent polyclonal tau antibody
Phospho-TDP43 immunohistochemistry revealed neuronal cytoplasmic inclusions in two of the seven cases. In one case, there was minimal involvement of the amygdala only. In the oldest subject, pTDP43 pathology extended beyond the amygdala into the mesial temporal cortex. No striatal pTDP43 or alpha-synuclein pathology was detected in any of our HD cases.
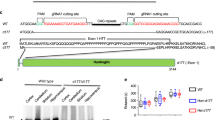
Given previous reports of an interaction of FUS, a major component of intranuclear inclusions in HD, with splicing factor proline- and glutamine-rich (SFPQ) in mediating exon 10 splicing of the tau gene, MAPT, we proceeded to explore the neuronal nuclear expression pattern of SFPQ in HD (Fig. 4). In neocortical sections, there was uniform and strong nuclear SFPQ reactivity in neurons. In striatal neurons, nuclear SFPQ expression was more variable, with some neurons being completely negative. However, no definitive intranuclear SFPQ aggregates were identified and no association was apparent between SFPQ nuclear signal intensity and presence or absence of HD-associated intranuclear inclusions.
Immunofluorescence staining for SFPQ (green) reveals strong nuclear neuronal reactivity in cortical sections a, but variable nuclear reactivity in the striatum d, g. SFPQ nuclear reactivity is diffuse and somewhat granular, but no intranuclear aggregates were identified. HD-associated intranuclear inclusions, highlighted by p62 (red) are seen in strongly a–f and weakly g–i SFPQ-positive cells.
Discussion
In this small case series, we systematically evaluated seven HD brains for the presence of age-associated neuronal and astroglial tau pathologies, applying for the first time the newly defined criteria for PART and ARTAG in the context of HD [28, 29]. We also describe the first case of CTE-like pathology in a HD patient.
The co-existence of neurofibrillary tangle and AD-like pathology in a subset of HD brains has long been recognized in a few single case reports and case series (summarized in ref. [26]). In the largest case review to date by Vonsattel et al., 13% of 1250 HD brains showed Alzheimer type changes insufficient in extent to meet neuropathological criteria of AD, and 2% of 866 brains had co-existing AD. As no details were provided, it is unclear how many of these case would now be considered PART [7]. In a more detailed evaluation of 27 HD brains aged 34–75 years by Jellinger et al., 33% of brains were found to be negative for tau and amyloid pathologies, 48% exhibited early tau pathology (Braak stages I–II) without associated amyloid deposition, and only 19% showed both amyloid and tau pathologies (Braak stages II–III) [22]. Using the current classification scheme, the group of tau-positive and amyloid-negative cases would meet criteria for definite PART. In a similar study focused on 15 elderly HD subjects, a higher proportion of cases (73%) exhibited AD neuropathologic changes, whereas only 13% of cases showed a PART pathology pattern [21], suggesting that the incidence of AD pathology increases with age in HD patients, similar to the general population. Although the small case number in our study precludes statistical analysis, amyloid-negative definite PART subjects were younger (mean age 60.3 years) than amyloid-positive subjects with possible PART or AD pathology (mean age 69.7 years), showing a similar trend.
Neuronal tau pathology in a pattern consistent with early Braak stages is a frequent occurrence in the aging brain [37]. Although Jellinger et al. commented that the tau pathology in their HD cohort occurred somewhat earlier than in their control group, case numbers were small and age ranges for both groups broadly overlapped with the age ranges for Braak stages I–II tau pathology in a large population cohort [37, 38]. There is currently no convincing evidence that the underlying HD pathogenic process accelerated or promoted the development of aging-related neuronal tau accumulation.
Tau does not only accumulate in neurons with aging, but also in astrocytes. While several earlier reports have recognized this phenomenon, the recent designation of a specific term for this process, ARTAG, has allowed for a more standardized evaluation and distinction from astroglial tau pathology occurring in primary tauopathies [29]. Using the specific morphological criteria proposed by Kovacs et al., we identified thorn-shaped astrocytes in three cases and granular/fuzzy astrocytes in one case. The overall ARTAG frequency of 57% in our HD cohort is higher than the reported frequency for control brains, but similar to the frequency in a wide range of neurodegenerative disorders, including AD and PART [30, 31]. Whereas the mean age at death of our HD cohort was more than a decade lower than in the AD/PART cohorts by Kovacs et al. [31], there was no difference in mean age between our ARTAG-positive and -negative cases and our cases numbers are too low to allow us to draw any conclusions about possible interactions between HD and ARTAG pathologies.
An unexpected finding was the presence of stage 1 CTE-like pathology in our youngest subject. Whereas this is the first description of CTE pathology in a HD patient, its co-existence has been reported in a wide range of neurodegenerative disorders [39,40,41], with the highest frequencies observed in progressive supranuclear palsy (PSP). Whereas most but not all of the positive cases had a history of contact sport participation or other form of traumatic brain injury, many patients also experienced multiple falls later in life related to their underlying neurodegenerative disease [41]. Our subject experienced frequent falls during his HD course, but no clinically symptomatic concussion event or other trauma history could be elicited in interviews with his family. Given the retrospective nature of our study, we were unable to obtain reliable data about falls in our other study subjects. If representative, the frequency of CTE-like pathology in our cohort is similar to the ones reported for aged controls and neurodegenerative conditions other than PSP, but the causative role of frequent falls in the development of CTE pathology needs further investigation, in particular in the setting of an underlying neurodegenerative process.
We did not identify any significant neuronal or glial tau pathology in the striatum, which aligns with the observations by Jellinger et al. [22] who did not find any subcortical neurofibrillary tangles or neuropil threads in their HD subjects, but is in contrast to the findings by Vuono et al. [24], who reported a wide range of neuronal and glial inclusions in the striatum and cortex of HD patients. They did not provide details about frequency and density of these aggregates in individual subjects, but noted, however, that the nucleus accumbens and inferior/anterior putamen were the sites of greatest tau pathology in the striatum. Given that these regions of the basal ganglia are least vulnerable to HD-associated degeneration on one hand [4, 5] and one of the subcortical brain regions more commonly affected by age-related tau deposition on the other hand [28], one might argue that the tau pathologies observed by Vuono et al. were aging-associated rather than HD-related. Furthermore, we were unable to detect any of the tau nuclear rods described by Fernandez-Nogales et al. at high frequencies in HD brains. This may be due to technical differences, as our tau antibodies showed much less neuronal cytoplasmic staining and the nuclear rods were thought to represent tau-filled nuclear indentations [23].
In apparent contrast to these inconsistent immunohistochemical findings of striatal tau deposition, several-independent studies have reported similar biochemical abnormalities in the striatum, most notably tau splicing alterations leading to an increased 4R/3R tau isoform ratio in HD [19, 23, 24]. There is only limited evidence of direct interaction between mutant huntingtin (mHtt) and tau in vivo [23, 25, 26], but the segregation of tau splicing factors by mHtt has been proposed as an indirect mechanism. Specifically, serine/arginine-rich splicing factor-6 (SRSF6), a putative tau splicing factor, was recently shown to accumulate in HD intranuclear inclusions and to increase 4R/3R tau ratio in cells co-transfected with mHtt and SRSF6 [23]. We propose an alternative mechanism mediated by the interaction of FUS with splicing factor, proline-, and glutamine-rich (SFPQ) based on several lines of evidence. Multiple groups have identified exon 10 in the tau gene, MAPT, as one of the principal exons that undergoes FUS-dependent alternative splicing in neurons [42,43,44,45,46]. Similarly, SFPQ has also been reported to be involved of MAPT exon 10 alternative splicing [47]. More recently, it was demonstrated that FUS and SFPQ interact in neuronal nuclei, and that loss of either functionality disrupts tau isoform equilibrium, increases the 4R/3R tau ratio, and induces pTau accumulation and neuronal loss in rodent models [48]. Whereas SFPQ nuclear reactivity was preserved in cortical neurons of our HD subjects, staining of striatal neurons was more variable, but no SFPQ intranuclear aggregates were identified. However, as described previously [9, 10], we found the HD-associated intranuclear inclusions to be strongly immunoreactive for FUS. In HD mice, it was shown that co-aggregation between FUS and mHtt resulted in the depletion of free FUS protein [49]. We therefore propose the hypothesis that the reduced nuclear availability of free FUS may be in part responsible for the observed tau isoform alterations in HD, possibly mediated through decreased interaction with SFPQ.
Finally, it should be pointed out that the observed biochemical changes in tau isoform ratio were more prominent at higher Vonsattel HD stages [19], when significant neuronal loss had already occurred and reactive gliosis is present. Analyses of neostriatal samples at later disease stages would therefore increasingly reflect the astrocytic component and possibly projections from remote neurons [5]. Although the astrocytic tau pathology in many but not all primary tauopathies as well as in ARTAG was shown to be 4R-predominant [29, 50], little is known about the tau isoform expression pattern by reactive astrocytes and may warrant further investigation.
In conclusion, in this small retrospective case series, we found evidence of tau hyperphosphorylation and aggregation in all of our HD cases. The tau pathology was readily classifiable into known diagnostic entities and most likely represents non-specific age-related changes. Compared to the age ranges reported for other cohorts, it does not appear that the PART and ARTAG pathologies were promoted or accelerated by the underlying HD processes, but confirmation in larger case series would be required. Several putative mechanisms have been put forth to explain the tau splicing abnormalities observed in HD brains, but their role in HD pathogenesis and progression are still undetermined and they do not necessarily promote immunohistochemically detectable pTau aggregates.
References
A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–83.
Kim SD, Fung VS. An update on Huntington’s disease: from the gene to the clinic. Curr Opin Neurol. 2014;27:477–83.
Sturrock A, Leavitt BR. The clinical and genetic features of Huntington disease. J Geriatr Psychiatry Neurol. 2010;23:243–59.
Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–84.
Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–77.
de la Monte SM, Vonsattel JP, Richardson EP Jr.. Morphometric demonstration of atrophic changes in the cerebral cortex, white matter, and neostriatum in Huntington’s disease. J Neuropathol Exp Neurol. 1988;47:516–25.
Vonsattel JP, Keller C, Del Pilar, Amaya M. Neuropathology of Huntington’s disease. Handb Clin Neurol. 2008;89:599–618.
DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–3.
Doi H, Okamura K, Bauer PO, Furukawa Y, Shimizu H, Kurosawa M, et al. RNA-binding protein TLS is a major nuclear aggregate-interacting protein in huntingtin exon 1 with expanded polyglutamine-expressing cells. J Biol Chem. 2008;283:6489–6500.
Woulfe J, Gray DA, Mackenzie IR. FUS-immunoreactive intranuclear inclusions in neurodegenerative disease. Brain Pathol. 2010;20:589–97.
Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–11.
Kwiatkowski TJ Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–8.
Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010;9:995–1007.
Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–31.
Neumann M, Roeber S, Kretzschmar HA, Rademakers R, Baker M, Mackenzie IR. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol. 2009;118:605–16.
Lagier-Tourenne C, Polymenidou M, Cleveland DW. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet. 2010;19:R46–64.
Charles V, Mezey E, Reddy PH, Dehejia A, Young TA, Polymeropoulos MH, et al. Alpha-synuclein immunoreactivity of huntingtin polyglutamine aggregates in striatum and cortex of Huntington’s disease patients and transgenic mouse models. Neurosci Lett. 2000;289:29–32.
Schwab C, Arai T, Hasegawa M, Yu S, McGeer P. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008;67:1159–65.
St-Amour I, Turgeon A, Goupil C, Planel E, Hébert SS. Co-occurrence of mixed proteinopathies in late-stage Huntington’s disease. Acta Neuropathol. 2018;135:249–65.
Tada M, Coon EA, Osmand AP, Kirby PA, Martin W, Wieler M, et al. Coexistence of Huntington’s disease and amyotrophic lateral sclerosis: a clinicopathologic study. Acta Neuropathol. 2012;124:749–60.
Davis MY, Keene CD, Jayadev S, Bird T. The co-occurrence of Alzheimer’s disease and Huntington’s disease: a neuropathological study of 15 elderly Huntington’s disease subjects. J Huntingt Dis. 2014;3:209–17.
Jellinger KA. Alzheimer-type lesions in Huntington’s disease. J Neural Transm. 1998;105:787–99.
Fernandez-Nogales M, Cabrera JR, Santos-Galindo M, Hoozemans JJ, Ferrer I, Rozemuller AJ, et al. Huntington’s disease is a four-repeat tauopathy with tau nuclear rods. Nat Med. 2014;20:881–5.
Vuono R, Winder-Rhodes S, de Silva R, Cisbani G, Drouin-Ouellet J, REGISTRY Investigators of the European Huntington's Disease Network, et al. The role of tau in the pathological process and clinical expression of Huntington’s disease. Brain. 2015;138:1907–18.
Blum D, Herrera F, Francelle L, Mendes T, Basquin M, Obriot H, et al. Mutant huntingtin alters Tau phosphorylation and subcellular distribution. Hum Mol Genet. 2015;24:76–85.
Gratuze M, Cisbani G, Cicchetti F, Planel E. Is Huntington’s disease a tauopathy? Brain. 2016;139:1014–25.
Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22.
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755–66.
Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 2016;131:87–102.
Kovacs GG, Robinson JL, Xie SX, Lee EB, Grossman M, Wolk DA, et al. Evaluating the patterns of aging-related tau astrogliopathy unravels novel insights into brain aging and neurodegenerative diseases. J Neuropathol Exp Neurol. 2017;76:270–88.
Kovacs GG, Xie SX, Robinson JL, Lee EB, Smith DH, Schuck T, et al. Sequential stages and distribution patterns of aging-related tau astrogliopathy (ARTAG) in the human brain. Acta Neuropathol Commun. 2018;6:50.
Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–2.
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11.
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13.
McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86.
McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64.
Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–9.
Braak H, Del Tredici K. Are cases with tau pathology occurring in the absence of Abeta deposits part of the AD-related pathological process? Acta Neuropathol. 2014;128:767–72.
Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto-Ortolaza A, Johnston AE, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130:877–89.
Koga S, Dickson DW, Bieniek KF. Chronic traumatic encephalopathy pathology in multiple system atrophy. J Neuropathol Exp Neurol. 2016;75:963–70.
Ling H, Holton JL, Shaw K, Davey K, Lashley T, Revesz T. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol. 2015;130:891–3.
Fujioka Y, Ishigaki S, Masuda A, Iguchi Y, Udagawa T, Watanabe H, et al. FUS-regulated region- and cell-type-specific transcriptome is associated with cell selectivity in ALS/FTLD. Sci Rep. 2013;3:2388.
Ishigaki S, Masuda A, Fujioka Y, Iguchi Y, Katsuno M, Shibata A, et al. Position-dependent FUS-RNA interactions regulate alternative splicing events and transcriptions. Sci Rep. 2012;2:529.
Lagier-Tourenne C, Polymenidou M, Hutt KR, Vu AQ, Baughn M, Huelga SC, et al. Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat Neurosci. 2012;15:1488–97.
Orozco D, Tahirovic S, Rentzsch K, Schwenk BM, Haass C, Edbauer D. Loss of fused in sarcoma (FUS) promotes pathological Tau splicing. EMBO Rep. 2012;13:759–64.
Rogelj B, Easton LE, Bogu GK, Stanton LW, Rot G, Curk T, et al. Widespread binding of FUS along nascent RNA regulates alternative splicing in the brain. Sci Rep. 2012;2:603.
Ray P, Kar A, Fushimi K, Havlioglu N, CHen X, Wu JY. PSF suppresses tau exon 10 inclusion by interacting with a stem-loop structure downstream of exon 10. J Mol Neurosci. 2011;45:453–66.
Ishigaki S, Fujioka Y, Okada Y, Riku Y, Udagawa T, Honda D, et al. Altered tau isoform ratio caused by loss of FUS and SFPQ function leads to FTLD-like phenotypes. Cell Rep. 2017;18:1118–31.
Kino Y, Washizu C, Kurosawa M, Yamada M, Doi H, Takumi T, et al. FUS/TLS acts as an aggregation-dependent modifier of polyglutamine disease model mice. Sci Rep. 2016;6:35236.
Kovacs GG, Lee VM, Trojanowski JQ. Protein astrogliopathies in human neurodegenerative diseases and aging. Brain Pathol. 2017;27:675–90.
Acknowledgements
The authors would like to acknowledge the excellent technical assistance of Jonette Werley, Mark Stauffer, and Kelly Ann Puglisi with immunohistochemical stains. The authors would also like to thank Peter Davies and Manuela Neumann for providing antibodies used in this project. This study was supported by the National Institute of Health grant NIA P50 AG005133 and the University of Pittsburgh Brain Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Baskota, S.U., Lopez, O.L., Greenamyre, J.T. et al. Spectrum of tau pathologies in Huntington’s disease. Lab Invest 99, 1068–1077 (2019). https://doi.org/10.1038/s41374-018-0166-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41374-018-0166-9
This article is cited by
-
Physiological expression of mutated TAU impaired astrocyte activity and exacerbates β-amyloid pathology in 5xFAD mice
Journal of Neuroinflammation (2023)
-
Tau: a biomarker of Huntington’s disease
Molecular Psychiatry (2023)
-
Huntington disease: new insights into molecular pathogenesis and therapeutic opportunities
Nature Reviews Neurology (2020)