Abstract
Objective
In 2017, our Level IV NICU switched from providing bovine-derived (BOV-fort) to human milk-derived fortifiers (HM-fort) and donor human milk (DHM) to premature infants born ≤ 30 weeks or ≤1250 g. Following this change, providers anecdotally observed increased hypoglycemia, hypercalcemia, and hyperphosphatemia. This study investigated potential laboratory differences between infants fed Bovine vs. Human milk derived fortifier.
Methods
Lab measurements from 402 infants (232 BOV-fort, 170 HM-fort) born between 2015 and 2019 were compared between groups.
Results
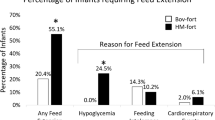
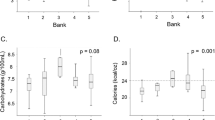
The proportion of infants ever having a blood glucose ≤ 45 mg/dL (p < 0.0001) was higher in the HM-fort group. The proportion of infants ever experiencing a phosphorus > 8.0 mg/dL were higher in the HM-fort group (p < 0.0001). The proportion of infants ever experiencing calcium > 11.4 mg/dL was higher in the HM-Fort group (p = 0.019).
Conclusions
Provision of HM-Fort and DHM to extremely premature infants is associated with metabolic derangements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bhatia J. Human milk and the premature infant. Ann Nutr Metab. 2013;62:8–14.
Morales Y, Schanler RJ. Human milk and clinical outcomes in VLBW infants: how compelling is the evidence of benefit? Semin Perinatol. 2007;31:83–8.
Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163:1592–5.e1.
Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kiechl-Kohlendorfer U, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156:562–7.e1.
Assad M, Elliott MJ, Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol. 2016;36:216–20.
Wickland J, Wade C, Micetic B, Meredith K, Martin G. A retrospective analysis of the effects of an exclusively human milk protein diet on neonatal feeding tolerance. Am J Perinatol. 2020;39:995–1000.
Jensen GB, Domellöf M, Ahlsson F, Elfvin A, Navér L, Abrahamsson T. Effect of human milk-based fortification in extremely preterm infants fed exclusively with breast milk: a randomised controlled trial. EClinicalMedicine. 2024;68:102375.
Staffler A, Klemme M, Mola-Schenzle E, Mittal R, Schulze A, Flemmer AW. Very low birth weight preterm infants are at risk for hypoglycemia once on total enteral nutrition. J Matern Fetal Neonatal Med. 2013;26:1337–41.
Chacham S, Pasi R, Chegondi M, Ahmad N, Mohanty SB. Metabolic Bone Disease in Premature Neonates: An Unmet Challenge. J Clin Res Pediatr Endocrinol. 2020;12:332–9.
Sethi A, Priyadarshi M, Agarwal R. Mineral and bone physiology in the foetus, preterm and full-term neonates. Semin Fetal Neonatal Med. 2020;25:101076.
Mitanchez D. Glucose regulation in preterm newborn infants. Horm Res. 2007;68:265–71.
Farrag HM, Cowett RM. Glucose homeostasis in the micropremie. Clin Perinatol. 2000;27:1–22.
Ackley D, Wang H, D’Angio CT, Meyers J, Young BE. Human milk-derived fortifiers are linked with feed extension due to Hypoglycemia in infants <1250 g or <30 weeks: a matched retrospective chart review. J Perinatol. 2023;43:624–8.
Enfamil Human Milk Fortifer Powder: Mead Johnson & Company, LLC; Available from: https://www.hcp.meadjohnson.com/s/product/a4R4J000000PpQiUAK/enfamil-human-milk-fortifier-powder.
Prolacta Human Milk Fortifier: Prolacta Bioscience; Available from: prolacta.com/en/products/preterm-nutrition-products/.
Liquid Protein Fortifier: Abbot Nutrition; Available from: https://abbottnutrition.com/liquid-protein-fortifier.
MCT oil Nestle Health Science Available from: https://www.nestlenutritionstore.com/mct-oilr.html.
Sharma A, Davis A, Shekhawat PS. Hypoglycemia in the preterm neonate: etiopathogenesis, diagnosis, management and long-term outcomes. Transl Pediatr. 2017;6:335–48.
Chang MR, Tetarbe M, Barton L, Ramanathan R, Cayabyab R. Transient hypoglycemia and biochemical differences in infants less than 1250 grams at birth fed human milk with human milk-derived fortifier versus cow milk-derived fortifier. Am J Perinatol. 2023. Epub ahead of print.
Amendoeira S, McNair C, Saini J, Habib S. Glucose homeostasis and the neonatal brain: a sweet relationship. Neonatal Netw. 2020;39:137–46.
Kerstjens JM, Bocca-Tjeertes IF, de Winter AF, Reijneveld SA, Bos AF. Neonatal morbidities and developmental delay in moderately preterm-born children. Pediatrics. 2012;130:e265–72.
Mank E, van Toledo L, Heijboer AC, van den Akker CHP, van Goudoever JB. Insulin concentration in human milk in the first ten days postpartum: course and associated factors. J Pediatr Gastroenterol Nutr. 2021;73:e115–9.
Rodel RL, Farabi SS, Hirsch NM, Rolloff KP, McNair B, Hernandez TL, et al. Human milk imparts higher insulin concentration in infants born to women with type 2 diabetes mellitus. J Matern Fetal Neonatal Med. 2022;35:7676–84.
Shehadeh N, Simmonds A, Zangen S, Riskin A, Shamir R. Efficacy and safety of enteral recombinant human insulin for reduction of time-to-full enteral feeding in preterm infants: a randomized, double-blind, placebo-controlled trial. Isr Med Assoc J. 2021;23:563–8.
O’Connor DL, Kiss A, Tomlinson C, Bando N, Bayliss A, Campbell DM, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial. Am J Clin Nutr. 2018;108:108–16.
Rowe J, Rowe D, Horak E, Spackman T, Saltzman R, Robinson S, et al. Hypophosphatemia and hypercalciuria in small premature infants fed human milk: evidence for inadequate dietary phosphorus. J Pediatr. 1984;104:112–7.
Koletzko B, Uauy R, Poindexter B. Nutritional care of preterm infants: scientific basis and practical guidelines. Nutr Care Preterm Infants. 2014;1:314.
Chetta KE, Hair AB, Hawthorne KM, Abrams SA. Serum phosphorus levels in premature infants receiving a donor human milk derived fortifier. Nutrients. 2015;7:2562–73.
Funding
This project was supported by the University of Rochester CTSA award number UL1 TR002001 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
DA and BY contributed to study conception and design, data collection, analysis and interpretation of results, and draft manuscript preparation. JY contributed to analysis and interpretation of results as well as draft manuscript preparation. JM and CD contributed to study conception, design and draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ackley, D., Yin, J., D’Angio, C. et al. Human milk derived fortifiers are associated with glucose, phosphorus, and calcium derangements. J Perinatol (2024). https://doi.org/10.1038/s41372-024-01977-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-01977-5