Abstract
Background
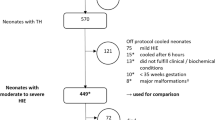
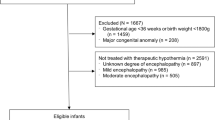
Infants at risk for hypoxic ischemic encephalopathy (HIE) require a time sensitive evaluation and decision-making regarding treatment with therapeutic hypothermia (TH). Prior to this project, there was no standardized approach to evaluating these infants locally.
Methods
Included infants were “at risk for HIE,” defined as meeting the “patient characteristics” and “biochemical criteria” per the institutional HIE pathway. Our primary outcome was documentation of an HIE therapeutic hypothermia evaluation (HIETHE) within the first six hours of life which included: (1) recognition of at-risk status, (2) an encephalopathy exam, and (3) a decision regarding TH. Plan-Do-Study-Act cycles included novel clinical decision support.
Results
From October 2020 to May 2023, among infants at-risk for HIE, the average percentage with an HIETHE documented improved from 47% to 82%.
Conclusions
We standardized the approach to infants at risk for HIE and improved the presence of a complete and timely evaluation regarding TH eligibility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–38.
Mortality GBD. Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84.
Committee on Fetus and Newborn, Papile LA, Baley JE, Benitz W, Cummings J, Carlo WA, et al. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133:1146–50.
Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166:558–66.
Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2013;2013:CD003311.
Flibotte J, Dysart K, Stoller J, Vossough A, Billinghurst L, Abend N, et al. ICU Clinical pathway for therapeutic hypothermia treatment for neonates with hypoxic ischemic encephalopathy (HIE). https://www.chop.edu/clinical-pathway/therapeutic-hypothermia-hypoxic-ischemic-encephalopathy-hie-clinical-pathway. 2021.
Carlton K, Cabacungan E, Adams SJ, Cohen SS. Quality improvement for reducing utilization drift in hypoxic-ischemic encephalopathy management. J Perinat Med. 2021;49:389–95.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42:377–81.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inf. 2019;95:103208.
Provost LP, Murray SK, Askews H. The health care data guide: learning from data for improvement. 1st ed. San Francisco: Jossey-Bass; 2011.
Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25:986–92.
Blecharczyk E, Lee L, Birnie K, Gupta A, Davis A, Van Meurs K, et al. Standardized evaluation of cord gases in neonates at risk for hypoxic ischemic encephalopathy. Hosp Pediatr. 2022;12:29–37.
Sittig DF, Wright A, Osheroff JA, Middleton B, Teich JM, Ash JS, et al. Grand challenges in clinical decision support. J Biomed Inf. 2008;41:387–92.
Author information
Authors and Affiliations
Contributions
Dr PJ. Peebles conceptualized and designed the study, collected the data, performed the data analysis and interpretation of data, and drafted the initial manuscript. Dr LH. Carr conceptualized and designed the study, built the clinical decision support tools in the electronic health record, participated in the interpretation of data, and critically reviewed and revised the manuscript. Dr. LP collected the data, performed the data analysis and interpretation of data, and critically reviewed and revised the manuscript. Drs. LC and JF conceptualized and designed the study, participated in the interpretation of data, and critically reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
Dr. Flibotte has received advisory board honoraria from Janssen Pharmaceuticals and Momenta Pharmaceuticals. The submitted work is unrelated to these activities. Dr. Carr is a partial owner of 2minutemedicine.com, a medical education and news website. The submitted work is unrelated to this company. The other authors have no relevant conflicts to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peebles, P.J., Christ, L., Flibotte, J. et al. Standardizing neonatal hypoxic ischemic encephalopathy evaluation and documentation practices. J Perinatol (2024). https://doi.org/10.1038/s41372-024-01916-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-01916-4