Abstract
Background
Recent studies have shown that obesity is associated with the severity of coronavirus disease (COVID-19). We reviewed clinical studies to clarify the obesity relationship with COVID-19 severity, comorbidities, and discussing possible mechanisms.
Materials and methods
The electronic databases, including Web of Science, PubMed, Scopus, and Google Scholar, were searched and all studies conducted on COVID-19 and obesity were reviewed. All studies were independently screened by reviewers based on their titles and abstracts.
Results
Forty relevant articles were selected, and their full texts were reviewed. Obesity affects the respiratory and immune systems through various mechanisms. Cytokine and adipokine secretion from adipose tissue leads to a pro-inflammatory state in obese patients, predisposing them to thrombosis, incoordination of innate and adaptive immune responses, inadequate antibody response, and cytokine storm. Obese patients had a longer virus shedding. Obesity is associated with other comorbidities such as hypertension, cardiovascular diseases, diabetes mellitus, and vitamin D deficiency. Hospitalization, intensive care unit admission, mechanical ventilation, and even mortality in obese patients were higher than normal-weight patients. Obesity could alter the direction of severe COVID-19 symptoms to younger individuals. Reduced physical activity, unhealthy eating habits and, more stress and fear experienced during the COVID-19 pandemic may result in more weight gain and obesity.
Conclusions
Obesity should be considered as an independent risk factor for the severity of COVID-19. Paying more attention to preventing weight gain in obese patients with COVID-19 infection in early levels of disease is crucial during this pandemic.
Similar content being viewed by others
Introduction
In December 2019, coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 infection has been emerged in Wuhan, China, and has spread rapidly and caused a global health crisis. By November 15, 2020, 53,766,728 confirmed cases of COVID-19, including 1,308,975 deaths, were reported to the World Health Organization [1] globally. COVID-19 manifestations include a spectrum of illnesses, ranging from asymptomatic infection [2] to severe pneumonia, adult/acute respiratory distress syndrome (ARDS), and even death [3]. This has caused medical researchers to identify risk factors related to the severity of COVID-19. Preexisting diseases such as hypertension (HTN), cardiovascular disease, diabetes, chronic respiratory disease, or cancer were identified as risk factors for developing severe COVID-19 [4,5,6]. However, recently, obesity was proposed as a significant independent risk factor for developing severe COVID-19 [7].
The obesity prevalence has recently increased in many developed and developing countries [8, 9], and has doubled in 73 countries since 1980 [10]. It is estimated that obesity prevalence is about 12% (603.7 million) among adults and 5% among children (107.7 million) worldwide [10]. This global increase in the prevalence of obesity shows the need for more assessment of this possible risk factor.
The effects of obesity on the respiratory system have been observed since long before. Avicenna [11], the famous Persian physician, recognized obesity as a medical disorder, and also in his book titled Canon of Medicine, referred to the respiratory problems of obese patients [12]. Also, the association between obesity and worse prognosis in the respiratory virus infections was observed in the “Spanish” influenza pandemic of 1918 [13]. Moreover, during the 2009 H1N1 pandemic, obesity was considered as an independent risk factor for severe disease or complications [14].
Surprisingly, level 1 evidence supports an obesity association with better prognosis in patients with ARDS [15]. However, previous studies have broken this paradox in COVID-19 patients [16,17,18]. These results and experiments with other viral respiratory infections reinforce the necessity of further research on this possible risk factor. Therefore, the aims of the present study were to: (1) review clinical studies in order to clarify the association between obesity and the severity of COVID-19, comorbidities and (2) discuss possible mechanisms.
Materials and methods
Search strategy and data collection
All studies conducted on COVID-19 and obesity were searched and reviewed. For this purpose, the electronic databases, including Web of Science, PubMed, Scopus, and Google Scholar, were searched. The search algorithm included all possible combinations of keywords from the following: “Severe acute respiratory syndrome coronavirus 2,” “COVID-19,” “2019-nCoV,” “SARS-CoV-2,” “coronavirus,” “obesity,” “weight,” “obese,” “body mass index,” and “adipose tissue.” Also, references of relevant review and editorial articles were reviewed to increase the coverage of included articles and ensure literature saturation.
All studies were independently screened by authors based on their titles and abstracts. The full texts of articles, potentially suitable for the review, were obtained to determine the relevance based on the study inclusion/exclusion criteria. The total of studies focusing on clinical characteristics and complications for SARS-CoV-2 was eligible for inclusion. The total of applicable studies (including case report, case series, and editorial, cross-sectional and cohort studies) was identified. We screened all reference lists of relevant studies in order to identify any missing publications. Studies that met the following criteria were included in the meta-analysis [1]: cohort studies; [2] body mass index (BMI) assessment ≥ 25 kg/m2 reported; [3] those indicating the odds ratio for the obesity risk [4]; age and gender were not kept as a bar for inclusion. The primary outcomes were the body weight, BMI, morbidities, and other outcomes. The data were extracted independently from the included studies by two authors based on a predefined data extraction sheet. The extracted data included (a) general information (author, type of study, and location), (b) participants (sample size, sex, and age), (c) outcomes (BMI, most common comorbidities, and mortality), and (d) main findings.
Any disagreements in the assessment of data were resolved by discussion between the two authors, and all potential discrepancies were resolved on consultation with a third reviewer. Forty relevant articles were selected, and their full texts were reviewed.
The meta-analysis was performed on outcomes with at least three studies conducted on their effects on obesity. All studies that evaluated the effect of the obesity on all outcomes, poor outcome, intensive care unit (ICU) admission, required IMV, and mortality were included in the meta-analysis. We used STATA Direct 3.1.22 to perform meta-analysis. A p value ≤ 0.05 was considered statistically significant. ORs were reported for the effect estimate, along with its 95% confidence intervals (CIs). Adjusted ORs (aORs) were pooled for meta-analysis of obesity and poor outcome, ICU admission, IMV, and mortality. Random-effects model was used for the data analysis, regardless of heterogeneity. The degree of heterogeneity was quantified using I2 statistics. I2 values of 25%, 50%, and 75% were considered to correspond to low, medium, and high levels of heterogeneity, respectively.
Results
Description of the included studies
After searching PubMed, Scopus, Web of Science, and Google Scholar databases, all relevant studies were identified. Finally, 55 articles were included in the study, and the full texts of the articles were reviewed. The characteristics of the included clinical trials and reasons behind the exclusion are summarized in Table 1.
All cohort, cross-sectional, descriptive, and retrospective studies were included in the study. Most countries that have conducted the studies on COVID-19 and obesity are China, the United States, Italy, UK, and France.
Obesity and COVID-19 severity and outcomes
We learned from the H1N1 Influenza epidemic that obesity is associated with more hospitalization, comorbidities, mechanical ventilation, and death [19, 20]. An increasing body of evidence shows that obesity influences severity and outcomes of COVID-19 (Table 1). A recent report released by the Intensive Care National Audit & Research Centre has shown that 39.3% of 9949 critically ill COVID-19 patients were obese, higher than the prevalence of 31.8% in the British age- and sex-matched general population. Also, the hazard ratio in survival analysis increased slightly above 1, when BMI rose above 30 kg/m2 [21].
Other studies also had similar results. A study conducted on 4103 COVID-19 patients in New York considered BMI > 40 kg/m2 as the most significant predictor of hospitalization after age [16]. Moreover, a study performed on 383 patients in China showed that obese patients had a 2.42-fold increased risk of severe pneumonia compared to normal-weight patients, even after adjusting for potential confounders [22]. Furthermore, Hajifathalian et al. [23] in a study conducted on 770 COVID-19 patients in New York found that patients with obesity were more likely to present overt symptoms and have twofold increased risk of ICU admission or death compared to normal-weight patients. Also, one study from China established that 88.24% of COVID-19 non-survivors had a BMI > 25 kg/m2 [24].
Mechanical ventilation, in addition to ICU admission, is considered as one of the most important indicators of severe disease. A small study in Seattle showed that 85% of obese patients need the mechanical ventilation [25]. Other studies confirmed the higher need for mechanical ventilation in obese patients. A study of 124 COVID-19 patients admitted in the ICU in a university hospital in Lille, France, reported a 7.36-fold need for intubation in patients with BMI > 35 (85.7% of them) compared to those with BMI < 25 kg/m2, independent of other comorbidities. Obesity (BMI ≥ 30) and severe obesity (BMI ≥ 35) were found in 47.6% and 28.2% of cases [17]. However, the results of another study in Lyon, France, were different. This study reported a lower prevalence of obesity and mechanical ventilation as compared to the Lille study. These differences may occur due to lower obesity prevalence in the Lyon area and different treatment protocols. However, the mechanical ventilation requirement was higher in obese patients (BMI ≥ 35 kg/m2) compared to lean patients (81.8 vs. 41.9%) [26]. Also, another study on 92 patients with COVID-19-associated pneumonia in Italy demonstrated that obesity is linked to a higher requirement of assisted ventilation (mechanical or noninvasive ventilation) and ICU admission, as two important indicators of disease severity, after adjusting for other variables. This study reported intensive or semi-intensive respiratory unit admission and mortality in 41.3 and 47.4% of obese patients. However, in this study, obese patients did not show significantly higher mortality rates [27].
Obesity and COVID-19 severity in age groups
Obesity could lead to severe COVID-19 both in young and old patients, but obesity in elderly individuals may be accompanied by more comorbidities, for example, obesity is associated with type-2 diabetes mellitus (T2DM) that is seen frequently in those aged 65 years [28]. Relative fat mass increases and muscle mass decreases in older individuals, even in nonobese ones, especially in patients with respiratory and cardiac diseases [29]. It was furthermore shown that risk of ICU admission in obese patients is higher than that in lean individuals across all age groups [23].
However, a study in New York showed that younger people with COVID-19 admitted to the ICU were obese [30]. Similarly, another study in New York demonstrated that people with a BMI between 30 and 34 kg/m2 and >35 kg/m2 were 1.8 and 3.6 times more likely to be admitted to the ICU, respectively. [18]. Moreover, in an Italian study, obese patients were ~9 years younger than normal-weight patients and needed more assisted ventilation and intensive or semi-intensive care [27]. It is essential to note that obesity may increase the chance of severe COVID-19 disease in younger individuals, especially in populations with high obesity prevalence.
Virus shedding in obese patients
Adipose tissue, as a viral reservoir, could make the virus shedding longer in obese patients. This pattern was also seen in influenza infection. More specifically this disease in obese patients was more contagious due to more prolonged shedding of virus [31], a lower interferon level [32], and a positive relationship of obesity with the virus load in exhaled air [33]. Viral shedding is also prolonged in obese patients due to impaired immune response, particularly reduced macrophage activation and impaired B- and T-cell responses [34].
Challenges in diagnosis and managing of obese patients
Many countries face a heavy burden of the COVID-19 pandemic on the health care system. Obesity and the heavy burden associated with COVID-19 have a two-sided relationship. Obese patients require more ICU beds [23], and due to the pandemic, many ICU beds are occupied. On the contrary, a high prevalence of obesity among a population could lead to the lack of ICU beds. Also, shortage of bariatric beds and the difficulty of positioning and transportation make more burden on nurses and other health care workers [7, 35].
Furthermore, appropriate requirements for obese patients are frequently scarce in many hospitals, e.g., computed tomography (CT) machines that are not able to accommodate obese patients’ weights. Besides these weight limits on CT machines, diagnosis based on pulmonary ultrasound is more difficult in obese patients, and these challenges make an early diagnosis more difficult in obese patients [36]. Also, difficult intubation due to additional adipose tissue on the larynx and difficult insertion of catheters are other factors that make managing obese patients so challenging [35, 36].
How obesity affects COVID-19?
Effects of obesity on the respiratory system
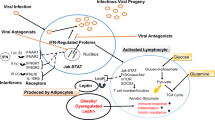
There are many hypotheses about the mechanism of how obesity affects COVID-19 (Fig. 1). It is not surprising that obese patients have many respiratory complications if infected with SARS-CoV-2. Obesity could affect the respiratory system in various ways. Previous studies have confirmed that obesity is a risk factor for many respiratory system diseases like asthma, obstructive sleep apnea syndrome, acute lung injury, and ARDS [37]. Frequently, there are alterations in the respiratory system and chest wall physiology of obese individuals due to fat deposits in the mediastinum and abdomen. Reduced chest wall elasticity, limited truncal expansion, low respiratory muscle strength, and limited diaphragm excursion predispose these individuals to lower respiratory system compliance with a decrease in expiratory reserve volume [10, 28, 38]. Obesity is associated with increased airway resistance, impaired gas exchange, positional gas trapping, and surfactant dysfunction [38, 39]. These functional and physiological changes predispose them to hypoventilation-associated pneumonia, pulmonary HTN, and cardiac stress [25].
Moreover, the impaired ventilation of the base of the lungs in obese patients could result in reduced oxygen saturation of the blood [40]. Lower lung zones have higher perfusion due to gravity; however, these zones are not well-ventilated because obese individuals’ functional residual capacity is near their residual volume [41,42,43]. This ventilation–perfusion mismatch could lead to lower partial pressure of oxygen (PO2) [40]. Furthermore, obese patients require more oxygen consumption to reach this lower PO2 [44]; thus, more oxygen requirement and hypoxia in obese patients could lead to the exacerbation of problems in oxygenation.
Effects of obesity on the immune system
Obesity could impair the immune system in various ways. From past experiences, we know that antibody response to influenza vaccine was reduced in obese individuals, and their infection risk was double than that of lean individuals [32, 45]. Moreover, in animal studies, the severity and duration of viral infection were increased in obese animals [32]. Also, obesity is an independent risk factor for an immune-mediated disease like psoriasis [46].
A balance between cytokines is necessary for an appropriate immune response. However, a pro-inflammatory state in obese patients disrupts this essential balance. This state may be due to the inappropriate secretion of adipokines and cytokines like tumor necrosis factor, interleukin-6 (IL-6), and C-reactive protein by adipocytes in hypoxic conditions [47, 48]. A previous study has shown that IL-6 is an independent risk factor for developing severe COVID-19 [49], and adipose tissue is one of the primary sources of IL-6 secretion [50]. The creation of an auto-regenerating inflammation loop in this pro-inflammatory state by recruitment of immune cells (macrophages, T cells, and B cells) impairs the immune system [48]. In this status, a viral infection could simply start an impaired immune response, leading to a cytokine storm by overproduction of pro-inflammatory cytokines. The cytokine storm could lead to vascular hyperpermeability and multiorgan failure seen in severe cases of COVID-19 [51]. In the human model, studies have shown that IL-6 is responsible for the activation of multiple cytokine pathways in this pro-inflammatory state [52].
Obesity could lead to the incoordination of innate and adaptive immune responses by disruption of lymphoid tissue integrity and alterations in leukocyte development and activity [36]. In particular, the impairment of CD8+ memory T cells was seen in obese patients vaccinated for influenza and considered as the leading cause of their weak antibody response to the vaccine [53]. A decrease in macrophage activation due to pro-inflammatory status was regarded as another cause of this inadequate response [34, 53]. Also, memory T-cell impairment could lead to more tissue damage during viral infection challenge [54]. Furthermore, a study in Wuhan showed that patients with severe COVID-19 had less memory, and regulatory T cells [22].
Leptin
Leptin is an adipose tissue-derived cytokine that signals the body to stop caloric consumption [35]. Also, leptin plays an essential role in the maturation, development, and function of B lymphocytes [55]. Leptin function in obese individuals is impaired. Surprisingly, leptin serum levels were very high in obese patients due to leptin resistance, like insulin resistance in T2DM [35], and this leptin resistance was associated with disease severity in H1N1 influenza [55].
Complement system
The complement system is inappropriately activated in obese patients [56]. This overreaction is one of the possible mechanisms of the impaired immune reaction to SARS-CoV-2. In a study conducted on SARS-CoV, C3 deficient mice had better respiratory function and a lower level of IL-6 [57].
Dipeptidyl peptidase-4 (DDP4)
Furthermore, a high level of DDP4 in obese patients is another possible mechanism of immune system impairment. DDP4 is a transmembrane enzyme in human adipose tissue and has an association with obesity-related T2DM [58]. DDP4 inhibition led to GLP-1 increasing and improved insulin sensitivity in adipose tissue [59]. Even in nonobese patients, DDP4 inhibition could suppress pro-inflammatory cytokines like IL-6 and IL-10 [60].
SARS-CoV-2 receptors in obese patients
Visceral adipose tissues like epicardial fat tissue and ectopic fat tissue in the alveolar space may play a significant role in severe disease by expressing a high level of angiotensin-converting enzyme 2 (ACE-2) receptors. SARS-CoV-2 has a high affinity to bind the ACE-2 receptors [61]. This receptor is expressed in many organs and tissue, particularly lung tissue [62]. However, adipose tissue has a higher level of ACE-2 expression than lung tissue [63]. Obese patients have more adipose tissue than lean individuals, resulting in more ACE-2 receptors.
Obesity and other comorbidities
Obesity could accompany other COVID-19 severity risk factors like T2DM, cardiovascular disease, and kidney diseases [25] (Fig. 2). In diabetic and obese patients, insulin resistance due to excess fat could lead to inadequate metabolic response to immunologic challenge and more insulin requirement during COVID-19 severe infection [29].
Even in the absence of other comorbidities, HTN, dyslipidemia, and insulin resistance could increase the risk of cardiovascular events in obese patients infected with SARS-CoV-2 [25]. Left ventricular (LV) hypertrophy due to HTN is the most common cardiac morphology change [64, 65]. In addition, the hyperactivity of the renin–angiotensin–aldosterone system in obese patients could affect myocardium by increasing levels of angiotensin II [66]. Furthermore, obesity could lead to right ventricular dysfunction as an issue in COVID-19 patients due to higher circulating plasma volume [51]. Also, LV diastolic dysfunction and heart failure are common in obese patients [64, 65, 67]. Moreover, the pro-inflammatory state in obese patients may increase the risk of thrombosis by creating an imbalance of procoagulant and anticoagulant factors, increasing thrombin generation, and enhancing platelet activation [68, 69]. Recently, in some patients with severe COVID-19 infection, a generalized thrombotic microvascular injury was observed in pulmonary and cutaneous biopsy and autopsy samples [70].
Vitamin D deficiency is another possible risk factor for developing severe COVID-19 [71]. Vitamin D as an immunomodulator plays an essential role in decreasing the production of pro-inflammatory cytokines [72]. About 40–80% of the obese population is vitamin D deficient [72] due to volumetric dilution, sequestration of vitamin D in adipose tissue, and negative feedback from 1,25-dihydroxy vitamin D [73]. Also, in the ICU, obese patients with malnutrition have worse outcomes than those without malnutrition [74]. Moreover, increasing the need for calorie consumption due to a severe acute inflammatory state in SARS-CoV-2 infection could provoke malnutrition [28].
Role of visceral and abdominal adipose tissue
Adipose tissues in each part of the body could play a role in the severity of COVID-19. For example, abdominal obesity is linked to the impaired ventilation, particularly in the supine state, by reducing diaphragmatic excursion [75]. There are two layers of adipose tissue in the abdomen: visceral and subcutaneous. Abdominal visceral fat is associated with T2DM, HTN, and cardiovascular disease [76]; however, a similar prevalence of T2DM to the USA in China may relate to more visceral and ectopic adipose tissue instead of abdominal fat in patients of Asian descent [7].
Visceral adipose tissue, either abdominal or non-abdominal, is responsible for a pro-inflammatory state by abnormal adipokine and cytokine secretion [54, 77]. This state makes visceral adipose tissue capable of driving inflammation in organs (such as heart, liver, kidney, and lungs) and also leads to immune response impairment [54]. Moreover, adipose tissue expresses a high level of ACE-2 receptors, and this may ease the local spreading of the virus in visceral intrathoracic (lungs), epicardial (heart), and peri-renal (kidney) adipose tissue [54]. H5N1 viral tropism has been shown for adipose tissue [78]. Also, adipose tissue is capable of more influenza virus shedding [31]. Moreover, visceral adipose tissue is one of the main sources of IL-6 secretion, and higher levels of IL-6 are associated with more mortality in SARS-CoV-2 [4].
Epicardial adipose tissue is another visceral fat tissue associated with increased BMI. This fat tissue could decrease myocardial function, in addition to adipokines secretion. Also, a high level of ACE-2 receptors in epicardial fat tissue could increase the risk of myocarditis in obese COVID-19 patients [79].
How COVID-19 lead to more obesity?
Social distancing during COVID-19 pandemic in many countries leads to significant changes in the individuals’ lifestyle. Decreasing activity, changing eating habits, sleep disorders, stress, and experiencing fear are some significant changes in people’s lives that may result in weight gain and obesity. Reduced level of daily physical activity may be due to the lockdown and keeping schools, gym, and parks closed, teleworking, depression, and fear.
On the other hand, stress and anxiety in people experiencing bad news may result in weight gain [80]. Stress could result in weight gain by cortisol release (activation of the hypothalamo-adrenal-pituitary axis) [81] and more fat and sweet food consumption (activation of reward centers in the brain) [82]. Also, stress is associated with sleep disruption and lower physical activity, resulting in weight gain [83].
Changing the diet to canned food due to food insecurity and fresh food unavailability, eating high-caloric snacks or junk foods during other activities and eating food late at night are unhealthy eating habits during the COVID-19 lockdown [81, 83]. These eating habits have more carbohydrate, fat, and sodium, and also processed food could lead to more food consumption by addictive-like behaviors. Furthermore, inadequate sleep may alter the circadian rhythm of hormones and immune system efficacy [83].
Moreover, self-isolation and depression are frequent in obese individuals due to weight stigma [84]. During the lockdown, there were lots of fat-shaming memes on social media [85], and this content could lead to more isolation. Depression or stigmatizing may lead to delay in referring to health care centers in obese patients [86].
Meta-analysis
Obesity and poor outcome
In 17 studies, meta-analysis demonstrated that obesity (BMI ≥ 30) was associated with poor outcome (OR: 1.297 [1.178–1.416], p < 0.001; I2: 0.0%, heterogeneity = 13.69) (Fig. 3A).
Obesity and ICU admission
In six studies, meta-analysis demonstrated that obesity (BMI ≥ 30) was not associated with ICU admission and obesity did not significantly increase the ICU admission (OR: 1.189 [0.955–1.424], I2: 0.0%, heterogeneity = 1.92) (Fig. 3B).
Obesity and IMV
In eight studies, meta-analysis suggested that in patients hospitalized with COVID-19, obesity (BMI ≥ 30) was associated with invasive mechanical ventilation (OR: 2.049 [1.420–2.678], I2: 75.3%, heterogeneity = 28.34) (Fig. 3C).
Obesity and mortality
Ten retrospective cohort studies reported the effect of obesity on the mortality. Meta-analysis demonstrated that obesity (BMI ≥ 30) was associated with mortality and obesity increased mortality (OR: 1.35 [1.241–1.459], I2: 76.6%, heterogeneity = 38.44) (Fig. 3D).
Recommendations on weight control in COVID-19 era
Because of obesity effects on COVID-19, weight gain prevention is highly recommended during the pandemic. Changing lifestyle has been found to play an important role in the prevention of weight gain. Increased physical activity and exercise at home or outdoors with social distancing are critical ways to prevent weight gain in normal-weight people and also lose weight in overweight or obese individuals. Exercise also could improve the metabolic and immunologic functions of the body [87]. Creative methods to do exercise more at home and high activity games with children may lead to more activity despite limitations. Reduced high-caloric food consumption is another feasible way to prevent weight gain. Increased fresh food and decreased canned and junk food consumption are notable points that should be considered during the pandemic. Moreover, psychological support and treatment or medical interventions to control stress and avoid emotional eating, and also, sleep regulation could also be useful to reach this goal.
In addition to these recommendations, obese patients should consider a steady low-caloric diet and proven weight loss drugs (synthetic or herbal drugs under physician observation) to lose weight due to their increased risk of disease [88, 89]. Furthermore, obese patients who have diabetes should tightly control their glycemic status and adjust their medication and calorie consumption with these new circumstances. As a result, telemedicine could play a crucial role in the close monitoring of obese patients during COVID-19 pandemic.
Recommendations and conclusion
Obesity affects the respiratory and immune systems through various mechanisms. Cytokine and adipokine secretion from adipose tissue leads to a pro-inflammatory state in obese patients, and this predisposes them to thrombosis, incoordination of innate and adaptive immune responses, inadequate antibody responses, and cytokine storm. Also, viral shedding is more in obese patients. Obesity is associated with other comorbidities such as HTN, cardiovascular disease, diabetes mellitus, and vitamin D deficiency.
Hospitalization, ICU admission, mechanical ventilation, and even mortality in obese patients are higher than normal-weight people. It seems obesity could shift severe COVID-19 symptoms to younger individuals. Also, early diagnosis and management of obese patients are harder than those of lean individuals. Decreasing activity, changing eating habits and, more stress and fear experienced during the COVID-19 pandemic may result in more weight gain and obesity. More attention to weight gain prevention is necessary during this pandemic.
Obesity should be considered as an independent risk factor for developing severe COVID-19. We recommend that clinicians consider more serious treatments in the early stages of disease for obese patients.
Reviewing all relevant studies conducted on “Obesity and COVID-19” and various possible mechanisms were the strength of this study. However, we have some limitations such as full-text unavailability for some non-English studies and lack of cohort studies. Most of the studies were cross-sectional and retrospective and also some had small sample sizes; we recommend that prospective cohort studies be conducted on larger sample sizes.
References
World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int. Accessed Nov 2020.
Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25. https://doi.org/10.2807/1560-7917.ES.2020.25.10.2000180.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62.
Mahbube E, Moloud P, Mahnaz Pejman S, Bagher L, Ebrahimpur M, Payab M, et al. Coronavirus disease (COVID-19): 10 questions and discussion points for diabetes and COVID-19. Adv J Emerg Med. 2020;0:118–23.
Parhizkar Roudsari P, Alavi-Moghadam S, Payab M, Sayahpour FA, Aghayan HR, Goodarzi P. et al. Auxiliary role of mesenchymal stem cells as regenerative medicine soldiers to attenuate inflammatory processes of severe acute respiratory infections caused by COVID-19. Cell Tissue Bank. 2020;21:405–25. https://doi.org/10.1007/s10561-020-09842-3.
Ryan DH, Ravussin E, Heymsfield S. COVID 19 and the Patient with Obesity - The Editors Speak Out. Obesity (Silver Spring). 2020;28:847. https://doi.org/10.1002/oby.22808.
Collaboration NCDRF, Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–96.
Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–724.
GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. https://doi.org/10.1056/NEJMoa1614362.
Avicenna. Al-Qanun fil-Tib. Cairo: Cairo Government Press; 1877, p. 304.
Nathan B, Cowan GSM. A medieval medical view on obesity. Obes Surg. 1992;2:217–8.
Short KR, Kedzierska K, van de Sandt CE. Back to the future: lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol. 2018;8:343.
Morgan OW, Bramley A, Fowlkes A, Freedman DS, Taylor TH, Gargiullo P, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS ONE. 2010;5. https://doi.org/10.1371/journal.pone.0009694.
Ni Y-N, Luo J, Yu H, Wang Y-W, Hu Y-H, Liu D, et al. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care. 2017;21:36.
Petrilli C, Jones S, Yang J, Rajagopalan H, O’Donnell L, Chernyak Y, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. BMJ. 2020;369. https://doi.org/10.1101/2020.04.08.20057794.
Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28:1195–9.
Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71:896–7.
Thompson DL, Jungk J, Hancock E, Smelser C, Landen M, Nichols M, et al. Risk factors for 2009 pandemic influenza A (H1N1)-related hospitalization and death among racial/ethnic groups in New Mexico. Am J Public Health. 2011;101:1776–84.
Louie JK, Acosta M, Winter K, Jean C, Gavali S, Schechter R, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. J Am Med Assoc. 2009;302:1896–902.
Intensive Care National Audit Research Centre. ICNARC report on COVID-19 in critical care 19 June 2020. London: Intensive Care National Audit & Research Centre; 2020.
Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020;43:1392–8.
Hajifathalian K, Kumar S, Newberry C, Shah S, Fortune B, Krisko T, et al. Obesity is associated with worse outcomes in COVID-19: analysis of early data from New York City. Obesity. 2020;28:1606–12.
Liu J, Ouyang L, Fu P, Cao Y, Yang D, Han X, et al. Epidemiological, clinical, radiological characteristics and outcomes of medical staff with COVID-19 in Wuhan, China: a single-centered, retrospective case series analysis. SSRN Electron J. 2020. https://doi.org/10.2139/ssrn.3555247.
Stefan N, Birkenfeld AL, Schulze MB, Ludwig DS. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16:341–2.
Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID-19 [letter]. Obesity. 2020;28:1175.
Busetto L, Bettini S, Fabris R, Serra R, Dal Pra C, Maffei P, et al. Obesity and COVID-19: an Italian snapshot. Obesity. 2020;28:1600–5.
Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5.
Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142:4–6.
Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395:1544–5.
Maier HE, Lopez R, Sanchez N, Ng S, Gresh L, Ojeda S, et al. Obesity increases the duration of influenza a virus shedding in adults. J Infect Dis. 2018;218:1378–82.
Honce R, Karlsson EA, Wohlgemuth N, Estrada LD, Meliopoulos VA, Yao J, et al. Obesity-related microenvironment promotes emergence of virulent influenza virus strains. MBio. 2020;11. https://doi.org/10.1128/mBio.03341-19.
Yan J, Grantham M, Pantelic J, De Mesquita PJB, Albert B, Liu F, et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Natl Acad Sci USA. 2018. https://doi.org/10.1073/pnas.1716561115.
Ahn SY, Sohn SH, Lee SY, Park HL, Park YW, Kim H, et al. The effect of lipopolysaccharide-induced obesity and its chronic inflammation on influenza virus-related pathology. Environ Toxicol Pharmacol. 2015;40:924–30.
Michalakis K, Ilias I. SARS-CoV-2 infection and obesity: common inflammatory and metabolic aspects. Diabetes Metab Syndr. 2020;14:469–71.
Muscogiuri G, Pugliese G, Barrea L, Savastano S, Colao A. Comentary: obesity: the “achilles heel” for COVID-19? Metab Exp. 2020;108. https://doi.org/10.1016/j.metabol.2020.154251.
Watanabe M, Risi R, Tuccinardi D, Baquero CJ, Manfrini S, Gnessi L. Obesity and SARS-CoV-2: a population to safeguard. Diabetes Metab Res Rev. 2020;36:e3325.
Murugan AT, Sharma G. Obesity and respiratory diseases. Chron Respir Dis. 2008;5:233–42.
Parameswaran K, Todd DC, Soth M. Altered respiratory physiology in obesity. Can Respir J. 2006;13:203–10.
Dixon AE, Peters U. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755–67.
Jones RL, Nzekwu MMU. The effects of body mass index on lung volumes. Chest. 2006;130:827–33.
Ladosky W, Botelho MAM, Albuquerque JP. Chest mechanics in morbidly obese non-hypoventilated patients. Respir Med. 2001;95:281–6.
Collet F, Mallart A, Bervar JF, Bautin N, Matran R, Pattou F, et al. Physiologic correlates of dyspnea in patients with morbid obesity. Int J Obes. 2007;31:700–6.
Fleischmann E, Kurz A, Niedermayr M, Schebesta K, Kimberger O, Sessler DI, et al. Tissue oxygenation in obese and non-obese patients during laparoscopy. Obes Surg. 2005;15:813–9.
Green WD, Beck MA. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc. 2017;14:s406–9.
Budu-Aggrey A, Brumpton B, Tyrrell J, Watkins S, Modalsli EH, Celis-Morales C, et al. Evidence of a causal relationship between body mass index and psoriasis: a mendelian randomization study. PLoS Med. 2019;16:1002739.
Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35.
Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cell Microbiol. 2014;16:1484–92.
Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–8.
Sindhu S, Thomas R, Shihab P, Sriraman D, Behbehani K, Ahmad R. Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: significance for metabolic inflammation. PLoS ONE. 2015;10:e0133494.
Jose RJ, Manuel A. Does coronavirus disease 2019 disprove the obesity paradox in acute respiratory distress syndrome? [letter]. Obesity. 2020;28:1007.
Yiu HH, Graham AL, Stengel RF. Dynamics of a cytokine storm. PLoS ONE. 2012;7:e45027.
Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity in mice reduces the maintenance of influenza-specific CD8+ memory T cells. J Nutr. 2010;140:1691–7.
Ryan PMD, Caplice NM. Is adipose tissue a reservoir for viral spread, immune activation, and cytokine amplification in coronavirus disease 2019? Obesity. 2020;28:1191–4.
Zhang AJX, To KKW, Li C, Lau CCY, Poon VKM, Chan CCS, et al. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207:1270–80.
Karkhaneh M, Qorbani M, Mohajeri-Tehrani MR, Hoseini S. Association of serum complement C3 with metabolic syndrome components in normal weight obese women. J Diabetes Metab Disord. 2017;16:49.
Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018;9:e01753–18.
Malavazos AE, Corsi Romanelli MM, Bandera F, Iacobellis G. Targeting the adipose tissue in COVID-19. Obesity. 2020;28:1178–9.
Iacobellis G. COVID-19 and diabetes: can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020;162:1–2.
Reinhold D, Brocke S. DPP4-directed therapeutic strategies for MERS-CoV. Lancet Infect Dis. 2014;14:100–1.
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–3.
Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7.
Jia X, Yin C, Lu S, Chen Y, Liu Q, Bai J, et al. Two things about COVID-19 might need attention. Preprints. 2020. https://doi.org/10.20944/preprints202002.0315.v1.
Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001;321:225–36.
Alpert MA, Lavie CJ, Agrawal H, Aggarwal KB, Kumar SA. Obesity and heart failure: epidemiology, pathophysiology, clinical manifestations, and management. Transl Res. 2014;164:345–56.
Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4:436–43.
Pascual M, Pascual DA, Soria F, Vicente T, Hernández AM, Tébar FJ, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–6.
Blokhin IO, Lentz SR. Mechanisms of thrombosis in obesity. Curr Opin Hematol. 2013;20:437–44.
Korakas E, Ikonomidis I, Kousathana F, Balampanis K, Kountouri A, Raptis A, et al. Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab. 2020;19:100250.
Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13.
Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients. 2020;12:988.
Teymoori-Rad M, Shokri F, Salimi V, Marashi SM. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29:e2032.
Walsh JS, Bowles S, Evans AL. Vitamin D in obesity. Curr Opin Endocrinol Diabetes Obes. 2017;24:389–94.
Robinson MK, Mogensen KM, Casey JD, McKane CK, Moromizato T, Rawn JD, et al. The relationship among obesity, nutritional status, and mortality in the critically Ill. Crit Care Med. 2015;43:87–100.
Dietz W, Santos-Burgoa C. Obesity and its implications for COVID-19 mortality [letter]. Obesity. 2020;28:1005.
Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–8.
Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes. 2013;37:333–40.
Gupte M, Thatcher SE, Boustany-Kari CM, Shoemaker R, Yiannikouris F, Zhang X, et al. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol. 2012;32:1392–9.
Zhao L. Obesity accompanying COVID-19: the role of epicardial fat. Obesity. 2020. https://doi.org/10.1002/oby.22867.
Rodríguez MÁ, Crespo I, Olmedillas H. Exercising in times of COVID-19: what do experts recommend doing within four walls? Rev Esp Cardiol. 2020;73:527–9.
Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE. 2015;10:e0117959.
Isasi CR, Parrinello CM, Jung MM, Carnethon MR, Birnbaum-Weitzman O, Espinoza RA, et al. Psychosocial stress is associated with obesity and diet quality in Hispanic/Latino adults. Ann Epidemiol. 2015;25:84–9.
Abbas AM, Fathy SK, Fawzy AT, Salem AS, Shawky MS. The mutual effects of COVID-19 and obesity. Obes Med. 2020. https://doi.org/10.1016/j.obmed.2020.100250.
Rubino F, Puhl RM, Cummings DE, Eckel RH, Ryan DH, Mechanick JI, et al. Joint international consensus statement for ending stigma of obesity. Nat Med. 2020;26:1–13.
Frühbeck G, Baker JL, Busetto L, Dicker D, Goossens GH, Halford JCG, et al. European association for the study of obesity position statement on the global COVID-19 pandemic. Obes Facts. 2020;2:292–6.
Alberga AS, Edache IY, Forhan M, Russell-Mayhew S. Weight bias and health care utilization: a scoping review. Prim Health Care Res Dev. 2019;20:e116.
Wong GCL, Narang V, Lu Y, Camous X, Nyunt MSZ, Carre C, et al. Hallmarks of improved immunological responses in the vaccination of more physically active elderly females. Exerc Immunol Rev. 2019;25:20–32.
Payab M, Hasani-Ranjbar S, Baeeri M, Rahimifard M, Arjmand B, Haghi-Aminjan H, et al. Development of a novel anti-obesity compound with inhibiting properties on the lipid accumulation in 3T3-L1 adipocytes. Iran Biomed J. 2020;24:155–63.
Payab M, Hasani-Ranjbar S, Shahbal N, Qorbani M, Aletaha A, Haghi-Aminjan H, et al. Effect of the herbal medicines in obesity and metabolic syndrome: a systematic review and meta-analysis of clinical trials. Phyther Res. 2020;34:526–45.
McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020;382:2005–11.
Liu M, He P, Liu HG, Wang XJ, Li FJ, Chen S, et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020. https://doi.org/10.3760/cma.j.issn.1001-0939.2020.03.014.
Liu J, Ouyang L, Guo P, Sheng Wu H, Fu P, liang Chen Y, et al. Epidemiological, clinical characteristics and outcome of medical staff infected with COVID-19 in Wuhan, China: a retrospective case series analysis. medRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.03.09.20033118v1.
Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–93.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020;23:2052–9.
Peng YD, Meng K, Guan HQ, Leng L, Zhu RR, Wang BY, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:e004.
Huang M, Yang Y, Shang F, Zheng Y, Zhao W, Luo L, et al. Early and critical care in severe patients with COVID-19 in Jiangsu Province, China: a descriptive study. SSRN Electron J. 2020. https://doi.org/10.2139/ssrn.3546056.
Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–52.
Xu Y, Xu Z, Liu XX, Cai L, Zheng H, Huang Y, et al. Clinical findings in critical ill patients infected with SARS-Cov-2 in Guangdong Province, China: a multi-center, retrospective, observational study. medRxiv. 2020:2020.03.03.20030668. https://doi.org/10.1101/2020.03.03.20030668.
McMichael TM, Clark S, Pogosjans S, Kay M, Lewis J, Baer A, et al. COVID-19 in a long-term care facility—King County, Washington, February 27–March 9, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–6.
Liao J, Fan S, Chen J, Wu J, Xu S, Guo Y, et al. Epidemiological and clinical characteristics of COVID-19 in adolescents and young adults. Innovation. 2020;1:100001.
Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York City [letter]. N Engl J Med. 2020;382:2372–4.
Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;21:2012–22.
Wu J, Li W, Shi X, Chen Z, Jiang B, Liu J, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med. 2020;288:128–38.
Khorrami Z, Nili S, Sharifi H, Eybpoosh S, Shokoohi M. Association of cigarette smoking, obesity, and underlying medical conditions with COVID-19 hospitalization and mortality in Iran: A nationwide retrospective ecological study. Med J Islam Repub Iran. 2020;34:133. https://doi.org/10.34171/mjiri.34.133.
Yu T, Cai S, Zheng Z, Cai X, Liu Y, Yin S, et al. Association between clinical manifestations and prognosis in patients with COVID-19. Clin Ther. 2020;42:964–72.
Bruno G, Perelli S, Fabrizio C, Buccoliero GB. Short-term outcomes in individuals aged 75 or older with severe coronavirus disease (COVID-19): first observations from an infectious diseases unit in Southern Italy. J Infect. 2020;81:e86–8.
Wei YY, Wang RR, Zhang DW, Tu YH, Chen CS, Ji S, et al. Risk factors for severe COVID-19: evidence from 167 hospitalized patients in Anhui, China. J Infect. 2020;81:e89–92.
Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical characteristics and outcomes of hospitalized and critically ill children and adolescents with coronavirus disease 2019 at a Tertiary Care Medical Center in New York City. J Pediatr. 2020;223:14–9.
Chen Q, Zheng Z, Zhang C, Zhang X, Wu H, Wang J. et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020;48:543–51.
Dreher M, Kersten A, Bickenbach J, Balfanz P, Hartmann B, Cornelissen C, et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int. 2020;117:271.
Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262.
de Lusignan S, Dorward J, Correa A, Jones N, Akinyemi O, Amirthalingam G, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–42.
Yu X, Sun X, Cui P, Pan H, Lin S, Han R, et al. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai, China. Transbound Emerg Dis. 2020;76:1697–707.
Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28:1200–4.
Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, et al. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease [letter]. Metabolism. 2020;108:154244.
Palmieri L, Vanacore N, Donfrancesco C, Lo Noce C, Canevelli M, Punzo O, et al. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. JGerontol A Biol Sci Med Sci. 2020;75:1796–800.
Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996.
Caussy C, Pattou F, Wallet F, Simon C, Chalopin S, Telliam C, et al. Prevalence of obesity among adult inpatients with COVID-19 in France. Lancet Diabetes Endocrinol. 2020;8:562–4.
Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a Children’s Hospital in New York City, New York. JAMA Pediatr. 2020;174:e202430.
Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, Vargas-Vázquez A, González-Díaz A, Márquez-Salinas A, et al. Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab. 2020;105:2752–61.
Yates T, Razieh C, Zaccardi F, Davies MJ, Khunti K. Obesity and risk of COVID-19: analysis of UK biobank [letter]. Prim Care Diabetes. 2020;14:566–7.
Buckner FS, McCulloch DJ, Atluri V, Blain M, McGuffin SA, Nalla AK, et al. Clinical features and outcomes of 105 hospitalized patients with COVID-19 in Seattle, Washington. Clin Infect Dis. 2020;71:2167–73.
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
Giacomelli A, Ridolfo AL, Milazzo L, Oreni L, Bernacchia D, Siano M, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158:104931.
Hu L, Chen S, Fu Y, Gao Z, Long H, Ren H, et al. Risk factors associated with clinical outcomes in 323 coronavirus disease 2019 (COVID-19) hospitalized Patients in Wuhan, China. Clin Infect Dis. 2020;71:2089–98.
Hur K, Price CPE, Gray EL, Gulati RK, Maksimoski M, Racette SD, et al. Factors associated with intubation and prolonged intubation in hospitalized patients With COVID-19. Otolaryngol Head Neck Surg. 2020;163:170–8.
Intensive Care National Audit & Research Centre. ICNARC report on COVID-19 in critical care 04 April 20. London: Intensive Care National Audit & Research Centre; 2020.
Itelman E, Wasserstrum Y, Segev A, Avaky C, Negru L, Cohen D, et al. Clinical Characterization of 162 COVID-19 patients in Israel: Preliminary Report from a Large Tertiary Center. Isr Med Assoc J. 2020;22:271–4.
Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity. 2020;29:1595–9.
Louisiana Coronavirus COVID-19 | Department of Health | State of Louisiana. https://ldh.la.gov/coronavirus/. Accessed Apr 2020.
Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14.
Ong SWX, Young BE, Leo YS, Lye DC. Association of higher body mass index (BMI) with severe coronavirus disease 2019 (COVID-19) in younger patients. Clin Infect Dis. 2020;71:2300–2.
Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract. 2020;14:295–300. https://doi.org/10.1016/j.orcp.2020.07.002.
Acknowledgements
Implementation of this study was sponsored by Tehran University of Medical Sciences (Endocrinology and Metabolism Research Center).
Author information
Authors and Affiliations
Contributions
SMMA, MP, and ME participated in the study design, drafting of the paper, and had significant role in development of the selection criteria and data extraction criteria. SMMA, BA, ZS, MPS, MQ, and BL contributed to the development of the selection criteria, data extraction criteria, and drafting of the paper. SMMA provided final approval of the version to publish. ZS, MPS, and MQ developed the search strategy. BL participated in critical review. MQ participated in the study design and interpretation. MP and ME supervised the project from scientific view of point and advised on experimental design. All authors read, provided feedback, and approved the final paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aghili, S.M.M., Ebrahimpur, M., Arjmand, B. et al. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: a review and meta-analysis. Int J Obes 45, 998–1016 (2021). https://doi.org/10.1038/s41366-021-00776-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00776-8
This article is cited by
-
Associations between COVID-19 incidence, weight status, and social participation restrictions in the U.S.: evidence from the national population, cross-sectional study
BMC Public Health (2024)
-
Liver injury in COVID-19: an insight into pathobiology and roles of risk factors
Virology Journal (2024)
-
Reduction of sugar, salt and fat content in foods over the period 2016–2021 in Spain: the National Food Reformulation Plan
European Journal of Clinical Nutrition (2024)
-
Association of excess visceral fat and severe illness in hospitalized COVID-19 patients in Japan: a retrospective cohort study
International Journal of Obesity (2024)
-
Effect of the SARS-CoV-2 pandemic on metabolic control in patients with type 2 diabetes: a 5-year cohort follow-up managed by a dynamic multidisciplinary team in Northeastern Mexico
Diabetology & Metabolic Syndrome (2024)