Abstract
Atopic dermatitis (AD) is characterized by skin barrier defects and increased interleukin (IL)-4/IL-13 expression. Recent evidence also suggests the involvement of innate immunity including Toll-like receptors, IL-33, IL-25, and innate lymphoid cells in the pathogenesis of AD. This article reviews these innate immune components and how they may become an integral part of prognostic factors and therapeutic targets in the treatment of AD.
Similar content being viewed by others
Main
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease of childhood. It affects up to 25% of children worldwide and 10–20% of children in the United States (1,2). AD is associated with food allergy, asthma, and allergic rhinitis. Patients with AD, particularly those with moderate to severe disease, are at increased risk for skin infections, sleep disorders, and psychosocial morbidities including depression and anxiety disorders (3,4). The cost of AD has been estimated to be as high as $3.8 billion per year in the United States (5). The cause of AD remains to be a subject of debate. Defects in the physical barrier of the skin have been proposed to play a primary role in the pathogenesis of AD (6). However, allergic inflammation, including increased expression of T helper (Th) 2 cytokines such as interleukin (IL)-4 and IL-13, may also induce skin barrier defects in AD (7). This review aims to summarize recent studies in the pathogenesis of AD.
Filaggrin Mutations: Evidence for Physical Barrier Defects as a Cause of AD
Filaggrin is an important skin barrier protein in the skin that functions in preventing water loss from the skin and intrusion by microbial pathogens and irritants (8). A breakdown product of filaggrin, natural moisturizing factor, acts in retaining moisture and further contributes to the hydration of the skin. Before the identification of filaggrin null mutations (FLG), decreased expression of filaggrin has been shown in AD (9). In 2006, Smith et al. and Palmer et al. identified FLG and showed significant association between FLG, ichthyosis vulgaris, and AD among European populations (reviewed in ref. 8). Since then, more than 30 independent studies worldwide have confirmed the association of FLG with AD. However, the majority of AD patients do not carry FLG. In children, FLG accounts for only 26.7% of the patients with AD, suggesting that other skin barrier genes, particularly those in the epidermal differentiation complex of chromosome 1q21, likely exist to account for the barrier defects in AD patients without FLG (10,11). Abnormalities in skin lipid composition (6), excessive serine protease activity (as illustrated by the ichthyosiform disease Netherton syndrome, which is caused by mutations in SPINK5 that encodes for lymphoepithelial Kazal-type–related inhibitor) (6), claudins of tight junction (12), or suppression of skin barrier protein expression by inflammation (9) may constitute other causes of skin barrier defects in AD. Mouse model of FLG has shown that deficiency of filaggrin in the skin leads to increased Th2 response and increased total serum immunoglobulin E (IgE) (reviewed in ref. 8). Multiple clinical studies have associated FLG with IgE sensitizations, asthma, and food allergy (reviewed in ref. 8). Using tape stripping and measurement of the concentrations of cytokines in the stratum corneum using a Luminex-based multiplex system, AD patients with FLG were determined to have increased IL-1 expression in their stratum corneum, as compared to AD patients without FLG (13). However, the mechanism(s) how FLG leads to Th2 inflammation in AD lesions remains unclear. A more recent human study showed that there was no significant difference in the basal ex vivo peripheral blood mononuclear cells expression of interferon (IFN)-γ or IL-4 between healthy controls, AD patients with or without FLG (14). All subjects were then sensitized to a nonallergenic chemical, 2,4-dinitrochlorobenzene, by epicutaneous application of 2,4-dinitrochlorobenzene. The 2,4-dinitrochlorobenzene-specific T-cell expression of IFN-γ and IL-4 was then analyzed. Healthy subjects were found to have significantly higher long-term expression of IFN-γ, whereas AD patients were found to have significantly higher IL-4 expression. Interestingly, there was no significant difference in the IL-4 expression between AD patients with or without FLG. This study suggests that FLG alone may not account for the pathogenesis of allergic inflammation in AD.
Keratinocyte Dysfunctions In AD
Keratinocytes, the key epithelial cells of the skin, are the primary cellular source of barrier deficiency in AD. In the past 10 years, there has also been progress in the understanding of keratinocytes in the immune dysregulation of AD. It has previously been shown that AD keratinocytes have an increased expression of granulocyte-macrophage colony-stimulating factor and TNF-α (15). Stimulated keratinocytes isolated from nonlesional skin of AD patients showed lower expression of human β defensin-2, an antimicrobial peptide that chemoattracts Th17 cells, as compared to that in healthy subjects and psoriasis patients (16). The lower antimicrobial peptide expression in AD lesions may contribute to the increased skin infections in these patients (17).
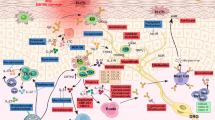
While the innate immune responses against microbial pathogens are downregulated in AD, there is an increased expression of thymic stromal lymphopoietin (TSLP), a pro-Th2 IL-7–like cytokine, in the keratinocytes of AD lesions (18). TSLP activates dendritic cells (DC) to produce chemokines such as thymus- and activation-regulated chemokine/chemokine (C-C motif) ligand (CCL)17 and macrophage-derived chemokine/CCL22, which leads to infiltration of Th2 cells in AD lesions (19). In a recent AD mouse model, TSLP was shown to activate cutaneous group 2 innate lymphoid cells (ILCs; Figure 1 ) (20). ILCs are characterized by their lymphoid morphology, but lack recombination-activating gene–dependent rearrangement antigen receptors and myeloid/dendritic cell markers (20). They are divided into three groups: group 1 ILCs are IFN-γ–producing cells that include natural killer cells; group 2 ILCs produce IL-13, IL-5, and/or IL-4; and group 3 ILCs produce IL-17 and IL-22 (21). Group 2 ILCs were shown to be significantly increased in human AD lesions, as compared to healthy skin (20).
Cutaneous immune response in atopic dermatitis. CCL, chemokine (C-C motif) ligand; D.C., dendritic cells; hBD-2, human β defensin-2; ILC, innate lymphoid cells; K.C., keratinocytes; MDC, macrophage-derived chemokine; S.C., stratum corneum; TARC, thymus- and activation-regulated chemokine; TSLP, thymic stromal lymphopoietin.
IL-33 and IL-25 are two other cytokines that may activate group 2 ILCs (22). IL-33 is an IL-1–related cytokine that induces allergic airway inflammation in mice in the absence of T and B cells (23). In addition, IL-33 has been shown to downregulate serum-induced expression of human β defensin-2 in keratinocytes (24). Immunohistochemical staining showed an increased number of IL-33+ cells among suprabasal keratinocytes and an increased staining of ST2, an IL-33 receptor, among dermal infiltrates in AD lesions (25). IL-25 increases the expression of IL-5 and IL-13 in TSLP-DC–activated T cells (26). Immunohistochemical staining showed increased IL-25+ keratinocytes in AD lesions, as compared to nonlesional skin (27). There was also an increased infiltration of cells, which expressed IL-17Rh1, an IL-25 receptor, in AD lesions, as compared to nonlesional AD skin (27). However, the majority of IL-25 producers are likely DC, eosinophils, and basophils (26,27).
Current evidence suggests that tissue repair mechanisms may underlie the pathogenesis of allergic inflammation (28). Double-stranded RNA released from damaged epithelial cells may stimulate Toll-like receptor (TLR) 3, leading to the production of TSLP from keratinocytes (29). Deletion of a disintegrin and metalloproteinase 17 (ADAM 17), a transmembrane metalloproteinase that cleaves cell surface proteins and maintains barrier homeostasis, in murine keratinocytes has been shown to result in skin barrier defects and chronic dermatitis in mouse models (30,31). Epidermal and systemic levels of TSLP and IL-33 were found to be significantly elevated in these mice (31). Epidermal expression of IL-4 and IL-17 was also found to be increased in ADAM17-deleted mice (31). The absence of ADAM17 in the mice results in a reduction of epidermal growth factor receptor and Notch signaling, which lead to increased expression of IL-33, and AP-1, TSLP, IL-4, and IL-13, respectively (30,31,32). However, increased expression of IL-17 is not a prominent feature of human AD lesions, but it is a characteristic of psoriatic lesions (33). In addition, homozygous mutation of ADAM17 in human leads to the development of inflammatory bowel disease, psoriaform dermatitis, and recurrent Staphylococcus aureus skin infections (34). Further studies are needed on the role of ADAM17 in AD.
Adaptive Immunity: Perpetrator of Persistent and Chronic Inflammation in AD
The role of adaptive immunity in the cutaneous inflammation of AD has been well established. T-cell expression of IL-4, IL-5, and IL-13 is significantly upregulated in both acute and chronic AD lesions, as compared to nonlesional AD skin and healthy skin (35,36). Based on atopy patch testing using house dust mite allergens, a biphasic model of cytokine expression by T cells in AD was observed: an initial increase in the infiltration of IL-4+ T cells into AD lesions, followed by infiltration of IFN-γ+ T cells in the chronic phase (37). T-cell activities in AD are directed by specialized DCs in the skin including epidermal Langerhans cells and inflammatory dendritic epidermal cells. These cells have increased expression of high-affinity receptor for IgE (FcεRI), which captures allergens for antigen processing and presentation to Th2 cells (38,39). TLR-activated DC determines the type of T-cell response: activation of TLR4 on DC by microbial pathogens induces Th1 response, whereas activation of TLR4 on DC in the presence of TSLP or TGF-β and IL-6 promotes Th2 or Th17 response, respectively (40). Langerhans cells isolated from healthy human skin induce T cells that produce less IFN-γ and IL-10, but more IL-4, IL-13, TNF-α, and thymus- and activation-regulated chemokine/CCL17 in the presence of TLSP (41).
IL-4 and IL-13 produced by Th2 have been shown to suppress filaggrin expression by keratinocytes (42). This is consistent with the clinical observation that filaggrin expression is significantly lower in the lesional AD, as compared to nonlesional AD skin (43). IL-4 and IL-13, together with TNF-α, also increase the expression of glucocorticoid-induced TNF receptor–related protein ligand in keratinocytes (44). Ligation of glucocorticoid-induced TNF receptor–related protein ligand on keratinocytes leads to the production of thymus and activation-regulated chemokine/CCL17, which further attracts Th2 cells to AD lesions. Cutaneous and systemic Th2 responses may amplify and maintain AD inflammation in a positive feedback mechanism (45). The absence of T-regulatory cells, and therefore a lack of suppression of cutaneous inflammation, may further contribute to the chronicity of AD (40).
Increased expression of IL-22 has been found consistently in both psoriatic and AD lesions (46,47). IL-22, together with Th2 cytokines and IL-31, may differentially induce keratinocyte differentiation proteins and epidermal hyperplasia (48). Its role in driving the development of psoriasis vs. AD may depend on the presence or absence of specific cytokines (49). More recently, Bromley et al. (50) showed that the chemokine (c-c motif) receptor 2–deficient mice which were injected intradermally with IL-23, a cytokine that increases the expression of IL-17 and IL-22 that characterize psoriatic lesions, developed skin lesions that resembled AD with elevated number of eosinophils and increased expression of IL-22, TSLP, and IL-4. The study did not find increased infiltration of Th2 cells in the lesions, suggesting that IL-22 may provide feedback to innate cells to increase allergic inflammation, rather than antimicrobial response, in chemokine (c-c motif) receptor 2–deficient mice. The role of chemokine (c-c motif) receptor 2 and IL-22 in human AD will require further studies. Both CD4+ T cells, which were induced by Langerhans cells and dermal DC (51), and CD8+ T cells, are the major contributors of IL-22 expression in AD lesions (47,52).
Role of Microbial Pathogens
S. aureus, Malassezia species, and Candida albicans are important triggers of cutaneous inflammation of AD (3,53,54). These microbial pathogens may induce host production of superantigen- or pathogen-specific IgE, which leads to basophil activation and histamine release (55). S. aureus cell wall products also bind to TLR, leading to the production of TSLP by keratinocytes (56). In addition to superantigens, S. aureus also produces α-toxin, which may be particularly virulent in filaggrin-deficient keratinocytes that lack sphingomyelinase, an enzyme that is required to cleave α-toxin receptor (57). α-toxin may also increase the risk of viral infections including herpes simplex virus and vaccinia virus in AD, resulting in eczema herpeticum and eczema vaccinatum, respectively (58).
The persistent colonization of S. aureus and Malassezia species may be influenced by epigenetics and microbiome, which are emerging areas of research in AD (59,60,61). The fungus Malassezia furfur has been shown to induce histone acetylation of antimicrobial peptide genes in keratinocytes (62). Bacterial components have been found in deeper skin compartments including the dermis, suggesting that the microbiome of the skin may extend beyond the skin surface (63). A mouse model showed that Staphylococcus epidermidis differentially upregulates the expression of IL-17 in the skin, rather than in the gut (64), suggesting a specific role of this bacteria in skin immunity. It has been shown that S. epidermidis may produce its own antimicrobial peptides that modulate the survival of other cutaneous microbial pathogens (65,66). More recently, Kong et al. (67) showed that increased bacterial diversity on typically affected skin areas of AD children was linked to treatments, whereas decreased bacterial diversity was associated with increased AD severity and flare. The decrease in bacterial diversity during AD flare corresponds to an increase in S. aureus and S. epidermidis sequences (67). Whether this increase in S. epidermidis during AD flare represents a compensatory and antagonistic mechanism against S. aureus will require further studies.
Clinical Implications and Challenges
In addition to therapeutic potential, studies of genetic, cellular, or cytokine markers may lead to early identification of different phenotypes of AD. This may be crucial in preventing morbidities such as infections, psychosocial issues, and respiratory allergies. Testing for these markers should be relatively simple (e.g., a blood test or noninvasive testing of stratum corneum) and inexpensive to be practical for clinical use. Elevated IgE have been associated with skin infections, respiratory allergy, and severity of AD (68,69). The presence and increased levels of specific IgE against microbial allergens correlate with AD severity (70,71). However, these markers tend to have a later onset (>3 y old) (72). Recent studies have associated FLG with AD severity, persistence, skin infections, and food allergy (8,73). Therefore, FLG may be a potential prognostic marker for clinical use. Genetic variations in TSLP are also a potential candidate for AD severity and herpes simplex virus infections (74). Further studies are also needed to delineate the pathways of innate immunity leading to the cutaneous inflammation of AD. The presence of early markers may allow for early intervention to prevent complications of AD. Mechanistic studies may also result in safer agents that target the innate immune response. An example is coal tar, which has long been known to be an effective treatment for AD (75). However, the potential carcinogenic effects and appearance of coal tar have hampered its clinical use in AD. More recently, it has been shown that coal tar may increase the differentiation of keratinocytes and expression barrier proteins including filaggrin via the activation of aryl hydrocarbon receptor (76). The study also showed that coal tar increased filaggrin expression in the keratinocytes of AD patients who were heterozygous for FLG. Further studies in this area may result in more effective and safer treatments for AD.
Conclusion and Future Directions
It is now well established that skin barrier defects are one of the primary causes of AD. FLG is first genetic marker of barrier defects that have been confirmed in the pathogenesis of AD. More recently, it has been shown that copy number variations of filaggrin genes may result in lower filaggrin expression and confer a risk for AD (77). Further studies are needed to search for therapies that increase filaggrin expression in these patients. However, since the majority of AD patients do not carry FLG, the search for other causes of AD is needed. Genetic variations may exist in other skin barrier molecules or in the cutaneous immune response of AD. Molecular or cellular components for microbial sensing such as TLR (78,79,80), or skin tissue repair such as TSLP and ILC (28), deserve further studies. Prospective clinical studies are needed to correlate laboratory markers with development of moderate to severe AD and complications of AD. Studies on early implementation of anti-inflammatory therapies or barrier repair are needed for the prevention of AD complications.
Statement of Financial Support
None declared.
Disclosure: none.
References
Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI ; ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009;124:1251–8.e23.
Shaw TE, Currie GP, Koudelka CW, Simpson EL . Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol 2011;131:67–73.
Ong PY, Leung DY . The infectious aspects of atopic dermatitis. Immunol Allergy Clin North Am 2010;30:309–21.
Chamlin SL, Chren MM . Quality-of-life outcomes and measurement in childhood atopic dermatitis. Immunol Allergy Clin North Am 2010;30:281–8.
Mancini AJ, Kaulback K, Chamlin SL . The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol 2008;25:1–6.
Elias PM, Hatano Y, Williams ML . Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol 2008;121:1337–43.
Irvine AD, McLean WH, Leung DY . Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011;365:1315–27.
Brown SJ, McLean WH . One remarkable molecule: filaggrin. J Invest Dermatol 2012;132(3 Pt 2):751–62.
Seguchi T, Cui CY, Kusuda S, Takahashi M, Aisu K, Tezuka T . Decreased expression of filaggrin in atopic skin. Arch Dermatol Res 1996;288:442–6.
Morar N, Cookson WO, Harper JI, Moffatt MF . Filaggrin mutations in children with severe atopic dermatitis. J Invest Dermatol 2007;127:1667–72.
Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol 2011;131:1644–9.
De Benedetto A, Rafaels NM, McGirt LY, et al. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol 2011;127:773–86.e1–7.
Kezic S, O’Regan GM, Lutter R, et al. Filaggrin loss-of-function mutations are associated with enhanced expression of IL-1 cytokines in the stratum corneum of patients with atopic dermatitis and in a murine model of filaggrin deficiency. J Allergy Clin Immunol 2012;129:1031–9.e1.
Newell L, Polak ME, Perera J, et al. Sensitization via healthy skin programs Th2 responses in individuals with atopic dermatitis. J Invest Dermatol 2013;133:2372–80.
Ong PY, Leung DY . Atopic dermatitis. Clin Allergy Immunol 2002;16:355–79.
de Koning HD, Kamsteeg M, Rodijk-Olthuis D, et al. Epidermal expression of host response genes upon skin barrier disruption in normal skin and uninvolved skin of psoriasis and atopic dermatitis patients. J Invest Dermatol 2011;131:263–6.
Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002;347:1151–60.
Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 2002;3:673–80.
Nakajima S, Igyártó BZ, Honda T, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol 2012;129:1048–55.e6.
Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 2013;5:170ra16.
Spits H, Artis D, Colonna M, et al. Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol 2013;13:145–9.
Cevikbas F, Steinhoff M . IL-33: a novel danger signal system in atopic dermatitis. J Invest Dermatol 2012;132:1326–9.
Oboki K, Ohno T, Kajiwara N, et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc Natl Acad Sci USA 2010;107:18581–6.
Alase A, Seltmann J, Werfel T, Wittmann M . Interleukin-33 modulates the expression of human ß-defensin 2 in human primary keratinocytes and may influence the susceptibility to bacterial superinfection in acute atopic dermatitis. Br J Dermatol 2012;167:1386–9.
Savinko T, Matikainen S, Saarialho-Kere U, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol 2012;132:1392–400.
Wang YH, Angkasekwinai P, Lu N, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med 2007;204:1837–47.
Hvid M, Vestergaard C, Kemp K, Christensen GB, Deleuran B, Deleuran M . IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J Invest Dermatol 2011;131:150–7.
Pulendran B, Artis D . New paradigms in type 2 immunity. Science 2012;337:431–5.
Vu AT, Chen X, Xie Y, et al. Extracellular double-stranded RNA induces TSLP via an endosomal acidification- and NF-?B-dependent pathway in human keratinocytes. J Invest Dermatol 2011;131:2205–12.
Franzke CW, Cobzaru C, Triantafyllopoulou A, et al. Epidermal ADAM17 maintains the skin barrier by regulating EGFR ligand-dependent terminal keratinocyte differentiation. J Exp Med 2012;209:1105–19.
Murthy A, Shao YW, Narala SR, Molyneux SD, Zúñiga-Pflücker JC, Khokha R . Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity 2012;36:105–19.
Dumortier A, Durham AD, Di Piazza M, et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS ONE 2010;5:e9258.
Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 2008;128:1207–11.
Blaydon DC, Biancheri P, Di WL, et al. Inflammatory skin and bowel disease linked to ADAM17 deletion. N Engl J Med 2011;365:1502–8.
Hamid Q, Boguniewicz M, Leung DY . Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest 1994;94:870–6.
Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY . In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol 1996;98:225–31.
Grewe M, Bruijnzeel-Koomen CA, Schöpf E, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today 1998;19:359–61.
Kraft S, Wessendorf JH, Hanau D, Bieber T . Regulation of the high affinity receptor for IgE on human epidermal Langerhans cells. J Immunol 1998;161:1000–6.
Wollenberg A, Kraft S, Hanau D, Bieber T . Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J Invest Dermatol 1996;106:446–53.
Novak N, Koch S, Allam JP, Bieber T . Dendritic cells: bridging innate and adaptive immunity in atopic dermatitis. J Allergy Clin Immunol 2010;125:50–9.
Ebner S, Nguyen VA, Forstner M, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells. J Allergy Clin Immunol 2007;119:982–90.
Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2009;124:3 Suppl 2:R7–R12.
Pellerin L, Henry J, Hsu CY, et al. Defects of filaggrin-like proteins in both lesional and nonlesional atopic skin. J Allergy Clin Immunol 2013;131:1094–102.
Byrne AM, Goleva E, Chouiali F, Kaplan MH, Hamid QA, Leung DY . Induction of GITRL expression in human keratinocytes by Th2 cytokines and TNF-a: implications for atopic dermatitis. Clin Exp Allergy 2012;42:550–9.
Reichle ME, Chen L, Lin SX, Chan LS . The Th2 systemic immune milieu enhances cutaneous inflammation in the K14-IL-4-transgenic atopic dermatitis model. J Invest Dermatol 2011;131:791–4.
Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R . IL-22 increases the innate immunity of tissues. Immunity 2004;21:241–54.
Nograles KE, Zaba LC, Shemer A, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 2009;123:1244–52.e2.
Gittler JK, Shemer A, Suárez-Fariñas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012;130:1344–54.
Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D . Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med 2010;207:1293–305.
Bromley SK, Larson RP, Ziegler SF, Luster AD . IL-23 induces atopic dermatitis-like inflammation instead of psoriasis-like inflammation in CCR2-deficient mice. PLoS ONE 2013;8:e58196.
Fujita H, Nograles KE, Kikuchi T, Gonzalez J, Carucci JA, Krueger JG . Human Langerhans cells induce distinct IL-22-producing CD4+ T cells lacking IL-17 production. Proc Natl Acad Sci USA 2009;106:21795–800.
Hijnen D, Knol EF, Gent YY, et al. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-?, IL-13, IL-17, and IL-22. J Invest Dermatol 2013;133:973–9.
Schmid-Grendelmeier P, Scheynius A, Crameri R . The role of sensitization to Malassezia sympodialis in atopic eczema. Chem Immunol Allergy 2006;91:98–109.
Faergemann J . Atopic dermatitis and fungi. Clin Microbiol Rev 2002;15:545–63.
Leung DY, Harbeck R, Bina P, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest 1993;92:1374–80.
Vu AT, Baba T, Chen X, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J Allergy Clin Immunol 2010;126:985–93, 993.e1–3.
Brauweiler AM, Bin L, Kim BE, et al. Filaggrin-dependent secretion of sphingomyelinase protects against staphylococcal a-toxin-induced keratinocyte death. J Allergy Clin Immunol 2013;131:421–7.e1–2.
Bin L, Kim BE, Brauweiler A, et al. Staphylococcus aureus a-toxin modulates skin host response to viral infection. J Allergy Clin Immunol 2012;130:683–691.e2.
Schauber J, Oda Y, Büchau AS, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol 2008;128:816–24.
Kong HH, Segre JA . Skin microbiome: looking back to move forward. J Invest Dermatol 2012;132(3 Pt 2):933–9.
Zhang E, Tanaka T, Tajima M, Tsuboi R, Nishikawa A, Sugita T . Characterization of the skin fungal microbiota in patients with atopic dermatitis and in healthy subjects. Microbiol Immunol 2011;55:625–32.
Angrisano T, Pero R, Paoletti I, et al. Epigenetic regulation of IL-8 and ß-defensin genes in human keratinocytes in response to Malassezia furfur. J Invest Dermatol 2013;133:2101–4.
Nakatsuji T, Chiang HI, Jiang SB, Nagarajan H, Zengler K, Gallo RL . The microbiome extends to subepidermal compartments of normal skin. Nat Commun 2013;4:1431.
Naik S, Bouladoux N, Wilhelm C, et al. Compartmentalized control of skin immunity by resident commensals. Science 2012;337:1115–9.
Iwase T, Uehara Y, Shinji H, et al. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010;465:346–9.
Gallo RL, Hooper LV . Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol 2012;12:503–16.
Kong HH, Oh J, Deming C, et al.; NISC Comparative Sequence Program. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012;22:850–9.
Simpson AB, Yousef E, Hossain J . Evaluation of the relationship between IgE level and skin superinfection in children with atopic dermatitis. Allergy Asthma Proc 2010;31:232–7.
Warner JA, McGirt LY, Beck LA . Biomarkers of Th2 polarity are predictive of staphylococcal colonization in subjects with atopic dermatitis. Br J Dermatol 2009;160:183–5.
Ong PY, Patel M, Ferdman RM, Dunaway T, Church JA . Association of staphylococcal superantigen-specific immunoglobulin e with mild and moderate atopic dermatitis. J Pediatr 2008;153:803–6.
Ong PY, Ferdman RM, Church JA . Association of microbial IgE sensitizations with asthma in young children with atopic dermatitis. Ann Allergy Asthma Immunol 2012;108:212–3.
Ong PY, Ferdman RM, Church JA . Late-onset of IgE sensitization to microbial allergens in young children with atopic dermatitis. Br J Dermatol 2010;162:159–61.
Cai SC, Chen H, Koh WP, et al. Filaggrin mutations are associated with recurrent skin infection in Singaporean Chinese patients with atopic dermatitis. Br J Dermatol 2012;166:200–3.
Gao PS, Rafaels NM, Mu D, et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J Allergy Clin Immunol 2010;125:1403–1407.e4.
SEDLIS E, PROSE P . Infantile eczema with special reference to the pathologic lesion. Pediatrics 1959;23:802–11.
van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest 2013;123:917–27.
Brown SJ, Kroboth K, Sandilands A, et al. Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose-dependent effect. J Invest Dermatol 2012;132:98–104.
Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K et al. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol 2004; 113:565–7.
Oh DY, Schumann RR, Hamann L, Neumann K, Worm M, Heine G . Association of the toll-like receptor 2 A-16934T promoter polymorphism with severe atopic dermatitis. Allergy 2009;64:1608–15.
Niebuhr M, Heratizadeh A, Wichmann K, Satzger I, Werfel T . Intrinsic alterations of pro-inflammatory mediators in unstimulated and TLR-2 stimulated keratinocytes from atopic dermatitis patients. Exp Dermatol 2011;20:468–72.
Acknowledgements
I wish to thank Rosemary Hernandez and Evelyn Ong for their assistance in the preparation of Figure 1.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Ong, P. New insights in the pathogenesis of atopic dermatitis. Pediatr Res 75, 171–175 (2014). https://doi.org/10.1038/pr.2013.196
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.196
This article is cited by
-
Evidence of pyroptosis and ferroptosis extensively involved in autoimmune diseases at the single-cell transcriptome level
Journal of Translational Medicine (2022)
-
Mast Cell-Specific MRGPRX2: a Key Modulator of Neuro-Immune Interaction in Allergic Diseases
Current Allergy and Asthma Reports (2021)
-
The Role of Yeast in Atopic Dermatitis Revisited: a Critical Appraisal
Current Dermatology Reports (2015)