Abstract
To study cardiovascular autonomic control, we assessed the effect of atropine on heart rate (HR) and blood pressure (BP) variability in 12 preterm infants (range 26–32 wk) before intubation for respiratory insufficiency. Spectral power analysis of R-R interval and systolic BP (SBP) series were estimated in a low-frequency (LF; 0.04–0.15 Hz) and high-frequency (HF; 0.4–1.5 Hz) band and evaluated for a 10-min period before and a 10-min period after atropine sulfate (0.01 mg/kg). Baroreceptor reflex (BR) functioning was estimated using transfer function analysis at LF (coherence, gain, and phase). Atropine resulted in a significant 12% increase in steady-state HR (p < 0.01) and unchanged SBP. For R-R interval series, the total spectral power decreased 6-fold (p < 0.01), which was predominantly due to a reduction in the LF band (16-fold; p < 0.01). In contrast, we observed a significant increase (25%; p < 0.05) in total spectral power of SBP series partly as a result of an increase in HF power. The LF power of SBP series was not altered. The median LF transfer gain (BR sensitivity) between SBP and R-R interval decreased from 4.2 to 1.4 ms/mm Hg (p < 0.01) after atropine. The LF phase relationship (BP leads R-R interval fluctuations by ∼4 s) was not changed after atropine. In conclusion, even in preterm infants in distress, atropine modulates HR and BP variability, suggesting that BR-mediated parasympathetic control of heart rate is of significance for cardiovascular control at that age.
Similar content being viewed by others
Main
The baroreceptor reflex (BR) is the most important regulatory mechanism in the short-term control of heart rate (HR) and blood pressure (BP). The BR buffers sudden changes in systemic BP by adapting HR and peripheral vascular resistance. These responses of HR and vascular resistance are mediated by the efferent parasympathetic and sympathetic limb of the BR. As both systems are involved, studies on the BR give information on autonomic cardiovascular regulation (1,2).
Data about the ontogeny of the BR and functional maturation in the human infant are limited. This is partly caused by the limited experimental (pharmacologic or mechanical) possibilities to challenge the BR in neonates. Passive head-up tilt test has been applied to neonates to measure responses in BP, HR, and limb blood flow to body tilting. In preterm infants (26–38 wk gestation), passive head-up tilt resulted in significant vasoconstriction of the lower limb with a slight fall in aortic BP and unchanged HR (3). The inadequate ability to maintain BP and the lack of tachycardia suggest that preterm infants lack the full integrated BR response as seen in adults. In term infants, however, a fall in systolic BP (SBP) was observed in conjunction with tachycardia and a fall in limb blood flow, suggesting the presence of active reflex vasoconstriction (4). In a recent study, BR maturation was studied longitudinally in preterm infants, using a beat-to-beat analysis of R-R interval series and noninvasively measured SBP (5). BR sensitivity (the R-R interval change in milliseconds per millimeter of mercury of SBP change) at birth was lower in preterm infants than in term infants and increased in postnatal life. This study, however, did not answer to what extent the parasympathetic and sympathetic system contribute to BR functioning. Previously, we showed the feasibility to use cross-spectral analysis to estimate BR functioning from spontaneous HR and BP fluctuations in stable preterm infants (6). With this technique, we found indications of a dominant role of the sympathetic system in stable preterm infants in the first days after birth.
Knowledge about the developmental aspects of the BR relies almost exclusively on animal studies. In animal studies, it is shown that the BR is impaired in the fetus and the newborn and develops further during postnatal life (7–9). The impairment in BR function is due to maturational changes in the autonomic pathways (10). In sheep, the sympathetic and parasympathetic control systems mature at different rates during fetal and neonatal development (11). The parasympathetic system exerts very little tone on the resting fetal HR before term gestation. Blockade of the parasympathetic system with atropine did not change HR in the premature fetuses. Only a significant HR increase was observed in the mature fetuses without significant changes in systemic BP (12). These observations suggest a progressive maturation of the parasympathetic nervous system during fetal development. In contrast to the negligible parasympathetic tone exerted on the fetal circulatory functions before maturity, the sympathetic control begins in early fetal life and increases with the progression of gestation (12). Blockade of the sympathetic system has profound effects on HR in all fetuses regardless of gestational age. In sheep, arterial BP and HR are depressed by ganglionic blockade in the newborn (1–3 d) but not in older lambs (13). These findings suggest that sympathetic tone is high during the transitory phase in the early postnatal period and decreases in later life.
In summary, human and animal data suggest that the development of the parasympathetic system parallels the maturation of BR function with gestational age and postnatal life. In addition, an activated sympathetic system is dominantly present in the immediate postnatal period and subsequently decreases over time. Whether vagal tone is able to modulate BR in sympathetically activated preterm infants in the transitory phase after birth is currently unknown. We therefore evaluated cardiovascular autonomic regulation by spectral power analysis in preterm infants in response to selective parasympathetic blockade. Atropine was administered as part of the standard neonatal intensive care management before endotracheal intubation for respiratory insufficiency.
The objective of this study was to determine the contribution of the parasympathetic system to BR functioning by comparing changes in spectral power of R-R interval and SBP series and changes in coherence, gain, and/or phase as assessed by transfer function analysis (between SBP and R-R interval fluctuations) before and after muscarinic blockade by atropine.
METHODS
Patients.
The study group consisted of 12 preterm infants. The relevant clinical data are shown in Table 1. Before labor, 10 women received antenatal steroids and nine women were on tocolytic (i.v. ritodrine) therapy. All infants were appropriate for gestational age, according to the Dutch growth charts (14). All infants were studied once, around the time of atropine administration just before the intubation procedure, in the first week of life. Administration of atropine sulfate (0.01 mg/kg intravenously) is part of our regular neonatal intensive care management before endotracheal intubation. The reason for endotracheal intubation was neonatal respiratory distress syndrome (n = 5), apnea (n = 5), and infection (n = 2). The median age of atropine administration was 36 h after birth. None had echoencephalographic evidence of cerebral hemorrhage or parenchymal lesions. Echocardiography revealed no structural abnormalities. In four patients, a patent ductus arteriosus was medically closed 30–120 h before the study. All infants were judged to be cardiovascularly stable without need for cardiotropic drugs (dopamine, dobutamine) or volume expanders at the time of the study. All patients' respiration was supported by nasal continuous positive airway treatment, with a mean airway pressure of 4 cm H2O. Four infants were on caffeine therapy with serum concentration levels between 10 and 20 mg/L, and all but three received antibiotic therapy. Informed consent was obtained from the parents of each infant. The local Ethics Committee approved the study.
Data acquisition and analysis.
A bipolar chest lead of the surface ECG and the transthoracic electric impedance waveforms were recorded by Hewlett Packard neonatal monitor type Merlin (Waltham, MA). The respiration rate was derived from the transthoracic electric impedance waveform. The arterial BP was measured invasively through a 4-French catheter in the lower aortic position used for routine monitoring of vital functions and intensive care management. A 0.5-mL/h infusion of heparinized physiologic saline solution was continuously flushed through the catheter. The sample frequency was 512 Hz for the ECG signals and 256 Hz for the arterial BP signals.
Recordings were made before the intubation procedure in the prone position. The total registration time was 30 min: 15 min baseline and 15 min after atropine. Figure 1 shows the traces of R-R interval and SBP series during the signal acquisition period in a patient. We divided the baseline period into three 5-min periods and found no significant differences in mean R-R interval or SBP between these periods. A 10-min period was selected from the first and second 5-min period to represent the control state and was subjected to spectral power analysis. Likewise, the post-atropine period was divided into three 5-min periods. After 5 min of atropine administration, no additional changes in R-R interval values were observed. The period 5–15 min after atropine administration was considered to be in a steady-state. These data were pooled to assemble one 10-min period to represent the atropine state and subjected to spectral power analysis.
Example of a 30-min trace of the R-R interval and SBP series in a preterm infant. The arrow (at time 15 min) indicates atropine administration. Note the decrease in the R-R interval and variability (upper trace) after atropine. In contrast, no change in SBP and variability (lower trace) is observed. The two boxes indicate two 10-min periods (control state and steady atropine state), which were used for further spectral power analysis.
Spectral power analysis of R-R interval series and SBP series.
With respect to details of the spectral power analysis, we refer to our previous report (6). Briefly, R waves were detected from the ECG, and an unevenly spaced R-R interval sequence was created. The R-R interval sequence was resampled at 4 Hz to obtain an equidistantly spaced time series. R-R artefacts were identified when the R-R interval was <200 or >500 ms. Missing R-R intervals were linearly interpolated. The amount of artefact reduction was <2% per patient. SBP was identified from peak detection of the arterial BP signal, resulting in an unevenly spaced “systogram.” The systogram was converted into an equidistantly spaced time series using the same resampling method as used for the R-R interval series. Spectral power of the various frequency components of SBP and R-R interval series was calculated using a moving fast Fourier transformation of 64-s segment length (256 points), providing a spectrum every 0.25 s with a resolution of 0.0156 Hz. Within each 10-min period (control state, post-atropine), the resulting spectra were subjected to assemble one average spectral density curve. The data acquisition software package was developed at the Department of Clinical Physics of our hospital in collaboration with the Department of Physics of the Eindhoven University of Technology, The Netherlands.
Unlike the consensus for spectral analysis in human adult (15), there is no agreement in spectral band definitions for neonatal studies (16–20). As the neonatal HR and respiration rates differ from that of the adult, neonatal studies likely require a different HF definition. Two frequency bands were defined, as indicated in Figs. 2 and 3: 1) the low-frequency (LF) band (0.04–0.15 Hz) reflecting BR activity and 2) the high-frequency (HF) band (0.4–1.5 Hz) reflecting the range of respiratory rate in preterm infants. The very low frequency band (0–0.04 Hz) was discarded to avoid possible contribution of slow trend artefacts. The total frequency band of interest was the range between 0.04 and 1.5 Hz. Spectral power was calculated in each defined frequency band. The values for spectral power are presented in units of milliseconds2 (for R-R interval series) and mm Hg2 (for BP series).
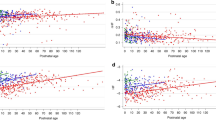
Change in spectral power for the R-R interval series after atropine administration. The individual spectral density curves and the (group) median spectral density curve of the R-R interval series are shown for the control state (A) and after atropine (B). The spectral power (milliseconds2) is distributed as a function of frequency (Hz). The x axis is logarithmic scaled to visualize the LF band better. The spectral density curves of the individual patients are presented as thin lines, and the median value is presented as a bold line. The vertical lines indicate the boundaries of the LF band (0.04–0.15 Hz) and HF band (0.4–1.5 Hz). Note that the spectral energy is particularly distributed in the LF and HF bands. In the LF band, individual peaks can be observed corresponding with BR activity. The increase of spectral power in the HF band corresponds to respiratory activity. The total spectral power is decreased 6-fold, predominantly in the LF band (16-fold) and much less in the HF band (3-fold).
Change in spectral power for the SBP series after atropine administration. The individual spectral density curves and the (group) median spectral density curve of the SBP series are shown for the control state (A) and after atropine (b). The spectral power (mm Hg2) is distributed as a function of frequency (Hz). The x axis is logarithmic scaled to visualize the LF band better. The spectral density curves of the individual patients are presented as thin lines, and the median value is presented as a bold line. The vertical lines indicate the boundaries of the LF band (0.04–0.15 Hz) and HF band (0.4–1.5 Hz). In the LF band, individual peaks can be observed corresponding with BR activity. The slight increase of spectral power in the HF band corresponds to respiratory activity. In contrast to the reduction in variability in the R-R interval series, total SBP variability is preserved after atropine and is even 25% higher after atropine.
Transfer function analysis.
In addition to the spectral density power, the transfer function analysis (coherence, transfer gain, and phase) was calculated, as previously described in detail (6,21,22). Briefly, transfer gain reflects the degree to which the input signal (SBP) amplitude becomes manifest in the output signal (R-R interval) amplitude at a discrete frequency. At LF, transfer gain is an estimate for BR gain/sensitivity (22,23). The phase difference (degrees or seconds) indicates the lead or lag of one signal with respect to the other at a discrete frequency. A negative phase relation indicates that SBP fluctuations lead R-R interval changes. In addition to these calculations, the coherence function was computed to assess the amount of linear coupling between SBP and R-R interval series in the frequency domain. The coherence function, which ranges from 0 (no relationship) to 1 (linear relationship), was used to assess the statistical reliability of the transfer function at each frequency. Transfer gain and phase were assessed in each frequency band at the frequency of highest coherence value (6).
Statistical analysis.
For each period, the autospectra densities were subjected to ensemble averaging. Because spectral power between patients does not display a normal distribution, the spectral power values of R-R interval and SBP series and transfer function analysis (frequency, coherence, gain, and phase) are expressed as (group) median and interquartile range (IQR). Comparisons between the control and atropine state were made with a two-sided paired Wilcoxon sign rank test for nonparametric data. The influence of gestational age and use of caffeine on spectral power values and transfer function variables was studied by linear regression analysis, using the method of least squares, and ANOVA. Statistical significance was accepted at p < 0.05.
RESULTS
The median R-R interval decreased from 422 to 378 ms (p < 0.01), equivalent to a 12% increase in HR after atropine. Table 2 shows the results of the spectral power values for R-R interval series and respiratory frequency in response to atropine administration. The median total spectral power decreased 6-fold from 135 to 22 ms2 after atropine (p < 0.01). Remarkably, the spectral power was much more decreased within the LF (16-fold; p < 0.01) than within the HF band (only 3-fold; p < 0.05). All patients showed a similar pattern with a decrease in total spectral power and a predominant decrease in LF. In Fig. 2, the individual spectral density curves and the (group) median spectral density curve of R-R interval series are shown for the control state (Fig. 2A) and after atropine (Fig. 2B). Atropine did not have a significant effect on the respiratory frequency of the patients (control state, median 0.98 Hz; atropine state, median 0.91 Hz). The respiratory frequency range was within the HF band. The median spectral LF/HF ratio decreased from 7.9 to 1.3 after atropine (p < 0.01).
SBP remained unchanged after atropine administration. Table 3 shows the results of spectral power values for SBP series in response to atropine administration. After atropine, total spectral power increased in nine patients. The median total spectral power increased from 4.1 to 5.2 mm Hg2 after atropine (25%; p < 0.05). Remarkably, no change in LF spectral power was observed. After atropine, the HF spectral power increased in 11 patients. The median HF spectral power increased from 1.4 to 1.7 mm Hg2 (20%; p < 0.01). In Fig. 3, the individual spectral density curves and the (group) median spectral density curve of SBP series are shown for the control state (Fig. 3A) and after atropine (Fig. 3B).
Table 4 shows the result of the transfer function analysis. The control state showed the highest coherence values (median 0.43; IQR 0.28–0.55) in the LF band at a median frequency of 0.08 Hz. The control state showed the highest coherence values in the HF band (median 0.73; IQR 0.62–0.82) at a median frequency of 0.81 Hz. In the control state, LF gain (median 4.2 ms/mm Hg) was lower than HF gain (median 8.3 ms/mm Hg; p < 0.05). In the control state, LF phase (median −3.7 s) was significantly different from the HF phase (median 0.4 s; p < 0.05).
The median LF transfer gain (BR sensitivity) decreased significantly from 4.2 to 1.4 ms/mm Hg after atropine (p < 0.01). The HF transfer gain did not change after atropine. In both frequency bands, atropine did not change the phase relation between SBP and R-R interval fluctuations. Because coherence values >0.5 are (arbitrarily) assumed to be of statistical significance using physiologic signals and might limit the value of the transfer function (23), we performed a subanalysis of the LF transfer function data in seven patients with coherence values >0.5. The median LF gain decreased significantly from 4.4 (IQR 3.1–5.7) to 1.0 (IQR 0.8–1.7) ms/mm Hg (p < 0.05). The median LF phase did not significantly change after atropine and remained at ∼−4 s. The (negative) phase relation in the LF band of the control state indicates that SBP fluctuations lead R-R interval changes. No significant influence could be demonstrated of gestational age and use of caffeine on R-R interval and SBP series, spectral power analysis, and transfer function analysis.
DISCUSSION
This study demonstrates clearly that vagal tone is present in the very first days after birth in preterm infants. Administration of atropine resulted in a significant increase in HR without altering SBP. Atropine profoundly decreased total variability of the R-R interval series, which was associated with a slight increase in total variability of SBP series. Remarkably, for R-R interval series, the decrease in LF power was larger than the decrease in HF power. The transfer function analysis showed a significant decrease in LF transfer gain (BR sensitivity) after atropine.
Endotracheal intubation is a commonly performed procedure in neonatal intensive care, and atropine is used as premedication to facilitate intubation of neonates (24). Atropine, a muscarinic receptor antagonist, exerts its influence on postganglionic muscarinic receptors of the sino-atrial node and atrioventricular node of the heart. Binding of acetylcholine to muscarinic receptors leads to a rapid (within 100 ms) membrane hyperpolarization (25). In addition, the effect of vagal impulse is brief because acetylcholine is rapidly hydrolyzed (25). Both characteristics of acetylcholine enable the parasympathetic system to be a fast-acting system. In contrast, cardiac responses to sympathetic stimulation arise relatively slowly (latency of 2–5 s) and dissipate over a longer period (25). It is assumed that LF fluctuations of the R-R interval and SBP are attributed to the BR and mediated by sympathetic as well as parasympathetic nerve activity. HF fluctuations of the R-R interval and SBP are associated with respiratory activity, and HF R-R interval fluctuations are mainly mediated through the (fast-acting) parasympathetic system (1,2,7,15,22,23,25).
HR and BP variability.
The observations that atropine increases HR and decreases its total variability are in agreement with findings from animal data as well as human data (26–32). The observation of unchanged SBP with increased SBP variability is confirmed by previous studies (28,29). Remarkably, we observed that at LF, the profound decrease in variability of the R-R interval series was not associated with an increase in variability of the SBP series. Apparently, at LF, the (slow-acting) sympathetic system is capable of adjusting SBP fluctuations. In contrast, the increase of SBP variability in the HF band after atropine suggests a loss of a (fast-acting) buffering system as a result of atropine. In preterm infants with respiratory distress syndrome and patency of the ductus arteriosus, it is possible that the increase in HR after atropine induces an increase in transductal shunting, which is known to be associated with an increase in HF BP variability (33). However, in the present study, none of the patients had ductal patency during the measurements.
The extreme thorax movements in patients who have severe respiratory distress and are breathing spontaneously may directly and mechanically exert profound oscillations in BP, which cannot be buffered completely by beat-to-beat variation in R-R intervals (34). Furthermore, one could speculate that in these patients, the respiratory fluctuations are faster than the frequency response of the heart to fluctuations in parasympathetic activity (35). Respiration may produce, via the BR, increasing and decreasing concentrations of acetylcholine at the sinus node. The response frequency of the sinus node, however, may be too slow to follow with acceleration and deceleration to these alternating concentrations of acetylcholine. This frequency-limiting step of the parasympathetic system might be related to the cellular properties of an immature parasympathetic nervous system or immature cardiac target cells. Parasympathetic innervation and acetylcholine esterase activity in the neonatal cardiac conduction system is only moderately present at birth, and a process of maturation presumably takes place later in life (36). This could explain why atropine showed less effect on the HF respiratory-associated R-R interval fluctuations. Three other explanations are possible for the small effect on HF fluctuations in the R-R interval series. First, the small effect of atropine on HF could be explained by the fact that HF is relatively low in sick preterm infants (6). Second, the study is not designed as a classical pharmacologic blockade study. The muscarinic receptor blockade is not validated for total blockade with agonists. However, we found in all patients a marked HR increase after atropine, and thus it seems unlikely that the dose was too small. Notably, we observed no initial “paradoxal” vagomimetic period, resulting from a low dose of atropine that can be seen in adults before the vagolytic effect (32). Third, the infants are in respiratory distress. The extreme thorax movements probably lead to direct stretching of the atria, not related to BR, resulting in respiratory-associated R-R interval fluctuations (37).
One of the interesting aspects of our study is the observation that atropine predominantly decreases the LF fluctuations and not the respiratory-associated HF fluctuations of the R-R interval series. These findings are in contrast with animal or human adult data. In adult rats, atropine increased HR and decreased the spectral HF peak (29,31). Similar results have been obtained in human adults, in which atropine increased HR and decreased HF spectral power (30,32,38). During sympathetic stimulation, variability of the R-R interval series showed virtually no spectral power in the HF band after atropine (30). In a study of thermoregulatory blood flow fluctuations in human adults, atropine resulted in a significant increase in HR. However, parasympathetic blockade caused a great reduction in power in all frequency bands in the HR spectrum, with remaining power in the LF band (39). Studies in healthy volunteers under controlled conditions (β-adrenoceptor blockade, metronomic breathing) show that the respiratory-associated fluctuations are more sensitive to slight changes in vagal activity than to tonic control (32). To explain these features, it can be hypothesized that difference in the shift of HR and respiratory-associated fluctuations are controlled by independent actions of the two primary medullary source nuclei of the vagus: the nucleus ambiguus and the dorsal motor nucleus. This hypothesis could fit with a phylogenetic theory named the polyvagal theory (40,41). Although vagal pathways from both nuclei terminate on the sinoatrial node, it is argued that the fibers that originate in the nucleus ambiguus are uniquely responsible for the phasic HR control, whereas the dorsal motor nucleus controls the neurogenic tonic HR (41). It is possible that the different vagal nuclei behave differently when exposed to atropine. Furthermore, it is likely that in preterm infants, the vagal system is not fully matured, has different myelination of the vagal nerve, or lacks the interconnections between the two vagal nuclei to ensure the (adult) phasic cardiorespiratory control (40,42).
The observation of a predominant effect on decreasing the LF spectral power of the R-R interval series after atropine indicates that the BR in preterm infants is under significant parasympathetic control. These vagal responses might reflect different physiology in the preterm infant, as discussed above. Several other explanations can be given for a predominant decrease in LF power of the R-R interval series. First, in addition to the dominant cardiac cholinergic (muscarinic) M2 receptor found in the adult heart, M1 subtypes may be found in the neonatal heart (43). These neonatal receptors may serve different physiologic responses to acetylcholine stimulation (44). Second, parasympathetic activity can modulate sympathetic effects by inhibiting norepinephrine release and modulating the sympathetic system (45). Likewise, blockade of the parasympathetic system might have an effect on sympathetic activity. Unknown is to what extent these interactions and processes are matured in preterm infants. Third, because breath amplitude modulation is associated with LF fluctuations in R-R interval series, the decrease of the LF component might be caused by a more stable respiration pattern after atropine administration (46).
In adults, the ratio low-to-high spectral power in the R-R interval series (LF/HF ratio) is assumed to reflect the “sympathovagal” balance (2,15). LF depends on sympathetic and parasympathetic activity, and HF is mediated by parasympathetic activity. This ratio has never been validated in neonates. Remarkably, we observed a significant decrease in the LF/HF ratio of the R-R interval series after atropine. Considering the predominant effect on decreasing the LF component of the R-R interval series after atropine and possible different physiology of cardiovascular regulation as discussed above, the LF/HF ratio might not be applicable in preterm infants in the transitory phase after birth.
Transfer function analysis and BR.
The control state in our study showed the highest coherence values within the LF band at ∼0.08 Hz, comparable with the natural frequency of the BR. In addition, we found in the control state an LF transfer gain (or BR sensitivity) of ∼4 ms/mm Hg. Of the total study group, nine patients showed a clear negative phase relation between SBP fluctuations and R-R interval changes, indicating a BR-mediated reflex: LF fluctuations in SBP lead R-R interval changes. In three patients, a slightly positive phase relation between SBP fluctuations and R-R interval changes was observed, possibly suggesting a feed-forward mechanism: LF changes in R-R interval lead fluctuations in SBP. No data are available in neonates on whether such a feed-forward mechanism exists. Alternatively, the circumstances during which the data are acquired (very preterm infants in respiratory distress, high arousal state) possibly limited the transfer function analysis. However, all patients of the subgroup analysis with coherence values >0.5 (assumed to be statistically more reliable) showed a negative phase relation, indicating a BR mechanism: LF fluctuations in SBP lead R-R interval changes by ∼4 s. These observations are in agreement with our previous report on BR functioning in preterm infants who have stable respiration (6).
We noticed after atropine administration a significant decrease in LF gain. LF gain is decreased because of the predominant effect of atropine on decreasing LF variability of the R-R interval series. LF phase relation did not change after atropine. This indicates that atropine changes the BR sensitivity but does not completely diminish the BR in preterm infants presumably because of sympathetic regulation. This aspect of decreased sensitivity is in agreement with human adult data in which atropine leads to a flattening of the cardiac baroreflex slope (47). In case of HR frequencies of 2.5 Hz and respiratory frequencies of ∼1 Hz, taking into account the closed-loop properties of the BR, interpretation of the HF phase shift is limited. At HF, we observed that a median phase between SBP and R-R interval fluctuations was close to 0 s with only small IQR (−0.3 to 0.5 s), indicating that SBP and R-R interval series are fluctuating together and probably not related to BR activity (29).
Methodologic considerations.
A limitation of the study group is its heterogeneity regarding the underlying pathology of the preterm infants. In contrast to our earlier study, we found in the present study lower coherence values of the LF transfer function analysis. The lower coherence values might be caused by acquisition “unselecting” data during the study. In contrast to our previous study, we analyzed much longer periods without taking the (quiet) sleep state into account. In addition, the character of the “acute” intervention (15 min of control state—atropine administration—15 min after atropine but before endotracheal intubation) precludes selecting such periods. For the same reason, we opted for a predefined HF band.
CONCLUSION
The present study shows that a significant vagal tone is already present in the transitory phase after birth. Atropine increases HR and decreases variability of the R-R interval series. In contrast to the R-R interval series, variability of the SBP series is preserved. Remarkably, atropine predominantly decreases the LF fluctuations of the R-R interval series. As a consequence, atropine decreases the LF transfer gain or BR sensitivity. These data show that atropine modulates HR and BP variability, suggesting that BR-mediated parasympathetic control of HR is of significance for cardiovascular control in the early postnatal period of distressed preterm infants.
Abbreviations
- BP:
-
blood pressure
- BR:
-
baroreceptor reflex
- HF:
-
high frequency
- HR:
-
heart rate
- IQR:
-
interquartile range
- LF:
-
low frequency
- SBP:
-
systolic blood pressure
References
Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ 1985 Hemodynamic regulation: investigation by spectral analysis. Am J Physiol 249: H867–H875
Malliani A, Pagani M, Lombardi F, Cerutti S 1991 Clinical and experimental evaluation of sympatho-vagal interaction: power spectral analysis of heart rate and arterial pressure variabilities I nGilmore JP, Zucker IH (eds) Reflex Control of the Circulation. CRC Press, Boca Raton, pp 1–194
Waldman S, Krauss AN, Auld PA 1979 Baroreceptors in preterm infants: their relationship to maturity and disease. Dev Med Child Neurol 21: 714–722
Picton-Warlow CG, Mayer FE 1970 Cardiovascular responses to postural changes in the neonate. Arch Dis Child 45: 354–359
Gournay V, Drouin E, Rozé JC 2002 Development of baroreflex control of heart rate in preterm and full term infants. Arch Dis Child Fetal Neonatal Ed 86: F151–F154
Andriessen P, Koolen AM, Berendsen RC, Wijn PF, ten Broeke ED, Oei SG, Blanco CE 2003 Cardiovascular fluctuations and transfer function analysis in stable preterm infants. Pediatr Res 53: 89–97
Shinebourne EA, Vapaavuori EK, Williams RL, Heymann MA, Rudolph AM 1972 Development of baroreflex activity in unanesthetized fetal and neonatal lambs. Circ Res 31: 710–718
Dawes GS, Johnston BM, Walker DW 1980 Relationship of arterial pressure and heart rate in fetal, newborn and adult sheep. J Physiol 309: 405–417
Altimiras J, Crossley DA II 2000 Control of blood pressure mediated by baroreflex changes of heart rate in the chicken embryo (Gallus gallus). Am J Physiol 278: R980–R986
Segar JL 1997 Ontogeny of the arterial and cardiopulmonary baroreflex during fetal and postnatal life. Am J Physiol 273: R457–R471
Assali NS, Brinkman CR 3rd, Woods JR, Dandavino A, Nuwayhid B 1977 Development of neurohumoral control of fetal, neonatal, and adult cardiovascular functions. Am J Obstet Gynecol 129: 748–759
Nuwayhid B, Brinkman CR 3rd, Su C, Bevan JA, Assali NS 1975 Development of autonomic control of fetal circulation. Am J Physiol 228: 337–344
Minoura S, Gilbert RD 1987 Postnatal change of cardiac function in lambs: effects of ganglionic block and afterload. J Dev Physiol 9: 123–135
Kloosterman GJ 1970 On intrauterine growth. Int J Gynaecol Obstet 8: 895–912
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. 1996 Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93: 1043–1065
Van Ravenswaaij-Arts C, Hopman J, Kollée L, Stoelinga G, Geijn H 1994 Spectral analysis of heart rate variability in spontaneously breathing very preterm infants. Acta Paediatr 83: 473–480
Spassov L, Curzi-Dascalova L, Clairambault J, Kauffmann F, Eiselt M, Medigue C, Peirano P 1994 Heart rate and heart rate variability during sleep in small-for-gestational age newborns. Pediatr Res 35: 500–505
Chatow U, Davidson S, Reichman BL, Akselrod S 1995 Development and maturation of the autonomic nervous system in premature and full-term infants using spectral analysis of heart rate fluctuations. Pediatr Res 37: 294–302
Mazursky JE, Birkett CL, Bedell KA, Ben-Haim SA, Segar JL 1998 Development of baroreflex influences on heart rate variability in preterm infants. Early Hum Dev 53: 37–52
Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Fifer WP, Myers MM 1999 Postural differences in cardiac dynamics during quiet and active sleep in low birth infants. Acta Paediatr 88: 1396–1401
Janssen BJ, Leenders PJ, Smits JF 2000 Short-term and long-term blood pressure and heart rate variability in the mouse. Am J Physiol 278: R215–R225
Head GA, Lukoshkova EV, Burke SL, Malpas SC, Lambert EA, Janssen BJA 2001 Comparing spectral and invasive estimates of baroreflex gain. IEEE Eng Med Biol Mag 20: 43–52
Robbe HW, Mulder LJ, Rüddel H, Langewitz WA, Veldman JB, Mulder G 1987 Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension 10: 538–543
Barrington KJ, Finer NN, Etches PC 1989 Succinylcholine and atropine for premedication of the newborn infant before nasotracheal intubation: a randomized, controlled trial. Crit Care Med 17: 1293–1296
Salata JJ, Zipes DP 1991 Autonomic nervous system control of heart rate and atrioventricular nodal conduction. In Gilmore JP, Zucker IH (eds) Reflex Control of the Circulation. CRC Press, Boca Raton, pp 67–101
Woods JR Jr, Dandavino A, Murayama K, Brinkman CR 3rd, Assali NS 1977 Autonomic control of cardiovascular functions during neonatal development and in adult sheep. Circ Res 40: 401–407
Goldberg JM, Moberg GP 1985 Autonomic control of heart rate in the neonatal rhesus monkey. J Med Primatol 14: 19–27
Ferrari AU, Daffonchio A, Albergati F, Mancia G 1987 Inverse relationship between heart rate and blood pressure variabilities in rats. Hypertension 10: 533–537
Cerutti C, Gustin MP, Paultre CZ, Lo M, Julien C, Vincent M, Sassard J 1991 Autonomic nervous system and cardiovascular variability in rats: a spectral analysis approach. Am J Physiol 261: H1292–H1299
Saul JPH, Berger RD, Albrecht P, Stein SP, Hui Chen M, Cohen RJ 1991 Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol 261: H1231–H1245
Japundzic N, Grichois ML, Zitoun P, Laude D, Elghozi JL 1990 Spectral analysis of blood pressure and heart rate in conscious rats: effects of autonomic blockers. J Auton Nerv Syst 30: 91–100
Medigué C, Girard A, Laude D, Monti A, Wargon M, Elghozi JL 2001 Relationship between pulse interval and respiratory sinus arrhythmia: a time- and frequency-domain analysis of the effects of atropine. Pflugers Arch 441: 650–655
Beuchée A, Pladys P, Senhadji L, Bétrémieux P, Carré F 2003 Beat-to-beat blood pressure variability and patent ductus arteriosus in ventilated, premature infants. Pflugers Arch 446: 154–160
Metsälä T, Siimes A, Antila K, Välimäki I 1993 Association of breathing movements to the variability of heart rate and blood pressure in foetal lambs. Acta Physiol Scand 147: 213–219
Stauss HM, Persson PB, Johnson AK, Kregel KC 1997 Frequency-response characteristics of autonomic nervous system function in conscious rats. Am J Physiol 273: H786–H795
Chow LT, Chow SS, Anderson RH, Gosling JA 1995 The innervation of the human myocardium at birth. J Anat 187: 107–114
Bernardi L, Keller F, Sanders M, Reddy PS, Griffith B, Meno F, Pinsky MR 1989 Respiratory sinus arrhythmia in the denervated human heart. J Appl Physiol 67: 1447–1455
Toska K, Eriksen M 1993 Respiration-synchronous fluctuations in stroke volume, heart rate and arterial pressure in humans. J Physiol (Lond) 472: 501–512
Lossius K, Eriksen M, Walloe L 1994 Thermoregulatory fluctuations in heart rate and blood pressure in humans: effect of cooling and parasympathetic blockade. J Auton Nerv Syst 47: 245–254
Taylor EW 1994 The evolution of efferent vagal control of the heart in vertebrates. Cardioscience 5: 173–182
Porges SW 1995 Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology 32: 301–318
Sachis PN, Armstrong DL, Becker LE, Bryan AC 1982 Myelination of the human vagus nerve from 24 weeks postconceptional age to adolescence. J Neuropathol Exp Neurol 41: 466–472
Pickoff AS 1998 Developmental electrophysiology in the fetus and neonate. In Polin RA, Fox WW (eds) Fetal and Neonatal Physiology. WB Saunders, Philadelphia, pp 891–913
Danilo P Jr, Rosen MR, Hordof AJ 1978 Effects of acetylcholine on the ventricular specialized conducting system of neonatal and adult dogs. Circ Res 43: 777–784
Randall WC, Randall DC, Ardell JL 1991 Autonomic regulation of myocardial contractility. In Gilmore JP, Zucker IH (eds) Reflex Control of the Circulation. CRC Press, Boca Raton, pp 39–65
Dykes FD, Ahmann PA, Baldzer K, Carrigan TA, Kitney R, Giddens DP 1986 Breath amplitude modulation of heart rate variability in normal full term neonates. Pediatr Res 20: 301–308
Parlow J, Viale JP, Annat G, Hughson R, Quintin L 1995 Spontaneous cardiac baroreflex in humans: comparison with drug-induced responses. Hypertension 25: 1058–1068
Author information
Authors and Affiliations
Corresponding author
Additional information
The current affiliation for R.C.M.B. is Department of Clinical Physics, Atrium Medical Center, PO Box 4446, 6401 CX Heerlen, The Netherlands.
Rights and permissions
About this article
Cite this article
Andriessen, P., Janssen, B., Berendsen, R. et al. Cardiovascular Autonomic Regulation in Preterm Infants: The Effect of Atropine. Pediatr Res 56, 939–946 (2004). https://doi.org/10.1203/01.PDR.0000145257.75072.BB
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000145257.75072.BB
This article is cited by
-
Vital signs as physiomarkers of neonatal sepsis
Pediatric Research (2022)
-
Early recognition of neonatal sepsis using a bioinformatic vital sign monitoring tool
Pediatric Research (2022)
-
Assessment of atropine-sufentanil-atracurium anaesthesia for endotracheal intubation: an observational study in very premature infants
BMC Pediatrics (2014)