Abstract
Mixed lineage kinase (MLK) 4, or MLK4, is a member of the MLK family of mitogen-activated protein kinase kinase kinases (MAP3Ks). Typically, MAP3Ks function to activate the mitogen-activated protein kinase (MAPK)-signaling pathways and regulate different cellular responses. However, here we report that MLK4β, unlike the other MLKs, negatively regulates the activities of the MAPKs, p38, c-Jun N-terminal kinase and extracellular signal-regulated kinase, and the MAP2Ks, MEK3 and 6. Our results show that MLK4β inhibits sorbitol- and tumor necrosis factor-induced activation of p38. Furthermore, MLK4β interacts with another MLK family member, MLK3, in HCT116 cells. Exogenous expression of MLK4β inhibits activation of MLK3 and also blocks matrix metalloproteinase-9 gelatinase activity and invasion in SKOV3 ovarian cancer cells. Collectively, our data establish MLK4β as a novel suppressor of MLK3 activation, MAPK signaling and cell invasion.
Similar content being viewed by others
Introduction
Mitogen-activated protein kinases (MAPKs) are serine/threonine kinases that regulate a variety of cellular processes including proliferation, inflammation, invasion and apoptosis.1, 2 The mammalian extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinase (JNK) and p38 kinase are three major MAPK families that are activated by a wide range of stimuli.3 ERK is primarily activated by growth factors, whereas JNK and p38 are activated by cytokines and stresses.2, 4 Upon stimulation, MAPKs activate cytosolic or nuclear-localized effector molecules and thereby translate the stimulus into a cellular response.3, 5, 6 The MAPKs are the executor kinases in a three-tiered kinase-signaling cascade. MAPKs are phosphorylated and activated by the mitogen-activated protein kinase kinases (MAP2Ks or MEKs), which are in turn phosphorylated and activated by the mitogen-activated protein kinase kinase kinases (MAP3Ks).2, 7, 8
Members of the mixed lineage kinase (MLK) family of serine/threonine kinases are MAP3Ks that activate the multiple MAPK-signaling pathways. The MLK family is divided into three subfamilies, the MLKs, dual leucine zipper-bearing kinases and zipper-sterile-alpha motif kinases.9 Of the four MLK subfamily members, MLK3 has been the best characterized in terms of its biological and biochemical function. MLK3 is required for tumor necrosis factor α (TNFα)-induced JNK activation in primary embryonic fibroblasts.10 In addition, mitogen- and cytokine-induced activation of B-Raf, ERK and JNK in lung and colon fibroblasts requires MLK3.11 MLK3 is also essential for JNK/AP-1-mediated breast cancer cell migration and invasion.12
In comparison to MLK3, much less is known about the biochemical and biological function of MLK4. In one study, it was reported that MLK4 is mutated in 6.8% of the colorectal cancers tested.13 However, another report indicated that MLK4 mutations were not observed in 46 colorectal cancer samples and were present in only 2 out of 24 different colorectal cancer cell lines (LoVo and CaR1).14 Moreover, no MLK4 mutations were detected in gastric and hepatocellular carcinomas.15 Therefore, the relationship between MLK4 mutation and cancer development remains elusive.
MLK4, similar to other MLKs, has an N-terminal SH3 domain, a kinase domain, a leucine zipper domain, Cdc42/Rac-interacting binding (CRIB) domain and a proline-rich C-terminus.9 There are two isoforms of MLK4, α (580 aa) and β (1036 aa), that are generated by alternative splicing. MLK4 shares >70% sequence homology with MLK3 in the kinase catalytic domain, which might suggest that MLK4, like MLK3, functions as an upstream activator of MAPK signaling.16 In the current study, we investigated the role of MLK4β in MAPK signaling. Here, we demonstrate that MLK4β overexpression, contrary to our expectations, reduces both the basal and stimulus-induced levels of active p38. Consistent with this finding, knocking down MLK4 expression increases basal and stimulus-induced p38, JNK and ERK activities. MLK4β overexpression in SKOV3 ovarian cancer cells also substantially reduced cell invasion and matrix metalloproteinase-9 (MMP-9) gelatinase activity. To elucidate potential mechanisms of MLK4β-mediated inhibition of MAPK activation and cell invasion, the effect of MLK4β expression on MLK3 activity was analyzed. MLK4β expression reduced MLK3 activity, whereas MLK4 knockdown increased MLK3 activity in HCT116 colon carcinoma cells. Moreover, an interaction between MLK4β and MLK3 was observed in HCT116 cells. Thus, our results indicate that MLK4β is a negative regulator of MLK3 and MAPK signaling and a suppressor of invasion in SKOV3 ovarian cancer cells.
Results
MLK4β negatively regulates p38 and MEK3/MEK6 activation
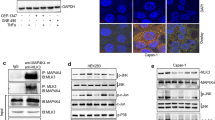
p38 is activated by stresses and pro-inflammatory cytokines.6 Once activated, p38 regulates a variety of cellular responses including invasion, inflammation and transformation.17, 18, 19 To test whether MLK4β, similar to other MAP3Ks, is an upstream activator of p38, we carried out small interfering RNA (siRNA)-mediated knockdown of MLK4 in HCT116 cells and analyzed the levels of active p38 using an activation state, phospho-specific p38 antibody (Thr180/Tyr182; phosphorylated-p38 (p-p38)). Surprisingly, MLK4-knockdown cells had elevated levels of p-p38 as compared with cells treated with a nonspecific siRNA oligo, while the total p38 levels remained unchanged (Figure 1a, left panel). External osmolarity conditions are critical for cell integrity and any changes, whether hypo- or hyperosmotic, induce osmotic stress that affects different cellular processes, including cell survival.20 The p38 pathway is activated in response to the hyperosmotic stress inducer sorbitol.21 Thus, HCT116 cells were treated with sorbitol to determine if MLK4 knockdown would affect p38 activation. Sorbitol treatment increased the level of p-p38 (Figure 1a, right panel). A further increase in the level of p-p38 was observed in the MLK4-knockdown cells as compared with cells treated with the nonspecific siRNA oligo (Figure 1a, right panel). The effect of ectopic expression of MLK4β on p38 activity was also analyzed in both untreated and sorbitol-treated cells. Overexpressing MLK4β almost completely abolished the level of p-p38 that was observed in untreated and sorbitol-treated cells transfected with an empty vector (Figure 1b). p38 signaling is also activated by cytokines.2, 6 Therefore, HCT116 cells were treated with the proinflammatory cytokine, TNFα, and the effect of MLK4β overexpression on p38 activity was analyzed. Similar to the results obtained with sorbitol, overexpressing MLK4β reduced the levels of TNFα-induced p-p38 (Figure 1c). MEK3 and MEK6 are MAP2Ks that specifically phosphorylate and activate p38.8, 22 To explore whether MLK4 has any effect on MEK3 and MEK6 activities, MLK4 was knocked down in HCT116 cells and the levels of active MEK3/MEK6 phosphorylated at serines 187 and 207 (p-MEK3/MEK6) were analyzed. p-MEK3/MEK6 and p-p38 levels were increased in cells with MLK4 knockdown as compared with cells treated with the nonspecific siRNA (Figure 1d). Similar results were obtained in ovarian epithelial carcinoma HEY1B cells (Figure 1e). Collectively, our results indicate that MLK4β inhibits basal, sorbitol- and TNFα-induced p38 activity and also negatively regulates the activation of MEK3/MEK6.
MLK4β negatively regulates p38 and MEK3/MEK6 activation. (a) HCT116 cells were transfected with nonspecific or MLK4 siRNA. The cells were left untreated (left panel) or treated (right panel) with 0.5 M sorbitol for 30 min and cell lysates were immunoblotted with MLK4β, p-p38, p38 and β-Actin antibodies. (b) HCT116 cells were transiently transfected with pCMV5-FLAG or FLAG-MLK4β and left untreated or treated with 0.5 M sorbitol for 30 min. Cell lysates were immunoblotted with FLAG, p38, p-p38 and actin antibodies. (c) HCT116 cells were transfected as described in b and left untreated or treated with 10 μM TNF-α for 20 min. Cell lysates were immunoblotted as described in b. HCT116 (d) and HEY1B (e) cells were transfected with nonspecific or MLK4 siRNA oligos. Cell lysates were prepared and immunoblotted with MLK4β, p-p38, p38, p-MEK3/MEK6, MEK3/MEK6 and β-Actin antibodies.
MLK4β, unlike MLK3, negatively regulates p38, JNK and ERK signaling
MLK3 activates MKK3 and MKK6, which in turn phosphorylate and activate p38.9 Our data thus far indicate that exogenous expression of MLK4β inhibits, rather than activates, p38. To determine if endogenous MLK4β has a similar effect on basal p38 activity, MLK4 was knocked down in HCT116 cells and basal p-p38 levels were analyzed. Silencing mlk4, but not mlk3, elevated the basal level of p-p38 (Figure 2a). Conversely, ectopic expression of MLK3 elevated the basal level of p-p38, whereas MLK4β expression reduced p-p38 levels, as compared with cells expressing the empty vector alone (Figure 2b). These data suggest that although MLK3 is an upstream activator of p38, MLK4β is a potent inhibitor of p38. Next, we tested the effect of MLK4β on the JNK and ERK pathway activation. Either MLK3 or MLK4 were knocked down in HCT116 cells, treated with sorbitol, and the levels of the active, phosphorylated JNK (p-JNK) and ERK (p-ERK) were analyzed. MLK3 knockdown did not affect the sorbitol-induced activity of any of the three MAPKs tested, whereas MLK4 knockdown increased the levels of not only p-p38 but also p-JNK and p-ERK, as compared with cells treated with nonspecific siRNA, suggesting that MLK4β also acts as a negative regulator of ERK and JNK activation (Figure 2c).
MLK4β negatively regulates p38, ERK and JNK signaling. (a) HCT116 cells were transfected with MLK3, MLK4 and nonspecific siRNA oligos. Cell lysates were immunoblotted with MLK4β, MLK3, p-p38, p38 and β-Actin antibodies. (b) Lysates from HCT116 cells transiently expressing pCMV5-FLAG, FLAG-MLK3 or FLAG-MLK4β were prepared and immunoblotted with FLAG, p-p38 and p38 antibodies. (c) HCT116 cells were transfected with nonspecific, MLK3 or MLK4 siRNA, and then treated with 0.5 M sorbitol for 30 min. Cell lysates were immunoblotted with MLK4β, MLK3, p-p38, p38, p-ERK, ERK, p-JNK, JNK and β-Actin antibodies.
MLK4β reduces p-MLK3 levels
Because MLK3 is an upstream activator of p38, JNK and ERK, we postulated that MLK4β might negatively regulate MAPK signaling by inhibiting MLK3 activity. To test this possibility, MLK4β was ectopically expressed in HCT116 cells and the level of active, phosphorylated MLK3 (p-MLK3) was analyzed. Interestingly, ectopic expression of MLK4β together with MLK3 completely abolished the levels of p-MLK3, suggesting that MLK4β inhibits MLK3 activation (Figure 3a). To confirm these results in another cell line, the effect of MLK4β on p-MLK3 levels in SKOV3 cells was analyzed. SKOV3 cells have a high basal level of p-MLK3 (Figure 3b). Ectopic expression of MLK4β significantly reduced the levels of p-MLK3 without affecting total MLK3 levels (Figure 3b). Furthermore, MLK4 knockdown in SKOV3 cells elevated the levels of p-MLK3 as compared with cells treated with the nonspecific siRNA, whereas the levels of total MLK3 remained unchanged (Figure 3c). Consistent with the results obtained for HCT116 cells, MLK4β expression also reduced p-p38 levels in SKOV3 cells (Figures 3b and c). These data reveal a novel inhibitory role for MLK4β in MLK3 activation.
MLK4β inhibits MLK3 activity. (a) Cell lysates were prepared from HCT116 cells overexpressing pCMV5-FLAG, FLAG-MLK3 or FLAG-MLK4β and immunoblotted with p-MLK3 and FLAG antibodies. (b) Cell lysates from SKOV3 cells transiently expressing pCMV5-FLAG or FLAG-MLK4β were immunoblotted with MLK4β, p-MLK3, MLK3, p-p38 and p38 antibodies. (c) SKOV3 cells were transfected with nonspecific or MLK4 siRNA and cell lysates were immunoblotted with MLK4β, p-MLK3, MLK3, p-p38, p38 and β-Actin antibodies.
MLK4β associates with MLK3
MLK4β and MLK3 share >65% sequence homology in all of the structural domains, including the leucine zipper domain, while the C-terminal domains are more divergent.16 To test if MLK4β interacts with MLK3, co-immunoprecipitations of glutathione S-transferase (GST)-tagged MLK3 and HA-tagged MLK4β were performed. In cells expressing both GST-MLK3 and HA-MLK4β, co-immunoprecipitation of GST-MLK3 with HA-MLK4β was observed (Figure 4a), suggesting that GST-MLK3 and HA-MLK4β interact in cells. The interaction between MLK3 and MLK4β was also observed in reciprocal experiments where GST-MLK3 was pulled down and immunoblotting was performed to detect HA-MLK4β (Figure 4b). In addition, co-immunoprecipitation of endogenous MLK3 and MLK4β was also observed (Figure 4c).
MLK4β associates with MLK3 and reduces SKOV3 cell invasion and MMP-9 activity. (a) HCT116 cells were transfected with pCMV5 vector, GST-MLK3, HA-MLK4β or both GST-MLK3 and HA-MLK4β. HA-MLK4β was immunoprecipitated from cell lysates with HA antibody. Co-immunoprecipitation of GST-MLK3 was assessed by immunoblotting with MLK3 antibody. HA-MLK4β was immunoprecipitated and immunoblotted with HA antibody to verify MLK4β expression. (b) A GST pull-down was performed with lysates from the cells described in a. The presence of HA-MLK4β and GST-MLK3 in the GST pull-downs was determined by immunoblotting with HA and GST antibodies, respectively. (c) Endogenous MLK4β was immunoprecipitated from HCT116 cell lysates and associated endogenous MLK3 was detected by immunoblotting with MLK3 antibody. The level of endogenous MLK4β in the immunoprecipitation was assessed by immunoblotting with MLK4β antibody. Control immunoprecipitations were performed with no antibody or rabbit IgG. (d) SKOV3 cells were transfected with pCMV5-FLAG or FLAG-MLK4β. Cell invasion was analyzed using Transwell chambers containing Matrigel. Cells that crossed the membrane were stained and counted (left panel). The experiment was repeated three times. Each bar represents the mean of data obtained from three independent experiments and the error bars represent deviation from the mean. Statistically significant differences from the control are indicated (*P<0.01). A portion of the cells was lysed and immunoblotted with FLAG and β-Actin antibodies to verify FLAG-MLK4β expression (right panel). (e) Culture media from the transfected cells described in d was subjected to gelatin zymography analysis.
MLK4β reduces cell invasion and MMP-9 activity in SKOV3 cells
Because MLK4β suppresses MLK3 activity, and MLK3 has recently been reported to regulate breast cancer cell invasion, we were interested in testing the effect of MLK4β on cell invasion.12 Expression of MLK4β in ovarian cancer SKOV3 cells (Figure 4d, right panel), which are highly invasive and have high basal levels of p-MLK3, resulted in significantly reduced cell invasion as compared with cells expressing the empty vector alone (Figure 4d, left panel). MMPs are proteolytic enzymes that promote cell invasion by breaking down components of the extracellular matrix.23, 24 To test whether MLK4β affects MMP-2 and MMP-9 enzyme activities, the levels of active MMP-2 and MMP-9 were analyzed in SKOV3 cell media by gel zymography. Although ectopic expression of MLK4β had little effect on MMP-2 gelatinase activity, it decreased the gelatinase activity of MMP-9 (Figure 4e). These results indicate that MLK4β expression reduces MMP-9 activity and blocks SKOV3 cell invasion.
Discussion
Functional characterization of MLKs other than MLK3 has been limited, and a thorough understanding of how MLKs 1, 2 and 4 function is necessary to identify redundancies and uncover unique functions of the MLK enzymes. Given that the MLKs share 75% homology in their catalytic kinase domains, it might be assumed that all MLKs activate MAPK signaling.16 However, here we report that MLK4β is a negative regulator of MAPK signaling. MLK4β inhibits both basal and sorbitol- and TNFα-induced p38 activity. In addition, MLK4β inhibits the p38 upstream activators, MEK3 and MEK6. A recent study reported that MLK4β overexpression failed to activate co-expressed p38 and that MLK4β overexpression reduced the levels of LPS-induced p38 activation 15 min post treatment.25 These observations support our findings that MLK4β functions to inhibit, rather than to activate, p38 activity. Our results also clearly demonstrate an elevation in the levels of active p38, JNK and ERK in sorbitol-treated cells when mlk4 is silenced, suggesting that the inhibitory role of MLK4 is not specific to p38, but extends to JNK and ERK as well (Figure 5).
A schematic diagram illustrating the role of MLK4β in MAPK signaling. MLK3, upon activation, activates the p38, JNK and ERK MAPK pathways leading to different cellular responses, including invasion. Our results indicate that MLK4β negatively regulates MLK3 activation and the ERK, JNK and p38 MAPK-signaling pathways. Therefore, MLK4β-dependent suppression of MAPK signaling may be mediated through inhibition of MLK3 activation.
MLK3 signaling promotes the activation of multiple MAPK pathways and stimulates cell invasion and migration.9, 12, 26 Thus, we proposed that MLK4β might exert its inhibitory effect on MAPK signaling by inhibiting MLK3 activity (Figure 5). Indeed, we found that MLK4β expression reduces the level of active MLK3 in HCT116 and SKOV3 cells, blocks SKOV3 cell invasion and reduces MMP-9 proteolytic activity.
Co-immunoprecipitation experiments revealed an association between overexpressed MLK4β and MLK3 and endogenous MLK4β and MLK3 in HCT116 cells, which to our knowledge is the first demonstration of an association between two MLK subfamily members. However, the nature of the interaction between MLK4β and MLK3, whether direct or indirect, and the domains involved remains to be determined. Because the leucine zipper domains of MLK4β and MLK3 do share 66% identity,16 heterodimerization between these two proteins remains a possibility.
MLK3 activity is autoinhibited by an interaction between the N-terminal SH3 domain and the proline residue 495, and autoinhibition is relieved upon binding of active Cdc42 to the MLK3 CRIB domain.9, 27 This binding promotes autophosphorylation and subsequent activation of MLK3. In a recent report, an interaction between MLK4β and Cdc42 was not detected.25 However, it is still possible that the association between MLK4β and MLK3 may interfere with Cdc42 binding to the MLK3 CRIB domain.
The MLK subfamily members are highly diverse in their C-terminal domains.9, 16 The fact that MLK3 and MLK4β, despite sharing >65% homology in all domains but the C-terminal domain, exhibit such drastic differences in their signaling suggests that the C-terminal domain may have a critical role in regulating MLK functions. Identification of the proteins that interact with the MLK C-termini is an interesting direction for future investigation.
Collectively, our results establish a novel role for MLK4β as a MLK3 inhibitor and a suppressor of MAPK signaling. Moreover, MLK4β expression blocks ovarian tumor cell invasion and MMP-9 activity, suggesting a potential function of MLK4β in suppressing metastatic dissemination of epithelial ovarian cancer cells.
Materials and methods
Cell lines and treatments
Human colon cancer (HCT116) and ovarian cancer (SKOV3) cells were obtained from the American Type Culture Collection (Manassas, VA, USA). The ovarian cancer cell line, HEY1B, was a gift from Dr Douglas Leaman (The University of Toledo). HCT116, SKOV3 and HEY1B cells were cultured in DMEM (Mediatech, Herndon, VA, USA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT, USA). All tissue culture media were supplemented with 25 μg/ml streptomycin and 25 IU penicillin (Mediatech). Cells were cultured in a humidified atmosphere with 5% CO2 at 37°C. For treatments, HCT116 cells were either treated with 0.5 M sorbitol (Fisher Scientific, Fairlawn, NJ, USA) for 30 min or with 10 nM of recombinant human TNF-α (BioSource, Camarillo, CA, USA) for 20 min.
Plasmids and siRNA transfections
The following expression vectors for human MLK3 and MLK4 were used in this study: pCMV5-FLAG-vector, pCMV5-FLAG-MLK3, pCMV-GST-MLK3, pCMV-HA-MLK3 and pCMV5-FLAG-MLK4β. Transient transfections were either performed using Lipofectamine (Invitrogen, Frederick, MD, USA) or PolyJet (SignaGen Laboratories, Rockville, MD, USA). Serum-free media was added to the cells prior to transfection and 5 and 10 μl of PolyJet or Lipofectamine reagent was used to transfect cells cultured in 6-cm and 10-cm dishes, respectively. The MLK3 siRNA oligonucleotide sequence has been previously described.11 The oligonucloeotide sequence of MLK4 siRNA is as follows: 5′-GGGCAGTGATGACTGAGAT-3′ and corresponds to nucleotides 1469–1487 in the MLK4-coding sequence. Non-targeting siRNA was formed (Dharmacon, Lafayette, CO, USA) and was used as a control. siRNA transfections were performed using Lipofectamine 2000, as previously described,11 or GeneMute (SignaGen Laboratories), as described by the manufacturer.
Immunoblotting and immunoprecipitation
Immunoblotting, cell lysis and immunoprecipitations were performed with the following antibodies: MLK3 (C-20), p38 (C-20), ERK (C-14), JNK (C-17) and β-Actin (C-4) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The MLK4 antibody specifically recognizes the C-terminus of MLK4β and does not cross-react with MLK4α (Novus Biologicals, Littleton, CO, USA). Activation-state p-p38 (Thr180/Tyr182), p-ERK (Thr202/Tyr204), p-JNK (Thr183/Tyr185), p-MLK3 (Thr277/Ser281) and p-MEK3/MEK6 (Ser189/207) antibodies were from Cell Signaling Technology (Beverly, MA, USA). FLAG antibody was from Stratagene (La Jolla, CA, USA). Anti-MEK6 antibody was from Stressgen Bioreagents corporation (Victoria, British Columbia, Canada).
Invasion assays
Cell-invasion assays were performed with 100 μl of 1 mg/ml BD Matrigel matrix (BD Bioscience, San Jose, CA, USA) in 24-well Transwells with 8.0-μm pore size and 24-mm diameter polycarbonate membrane (Corning, Acton, MA, USA). Cells were washed in DMEM containing 0.5% fetal bovine serum. In all, 10 000 cells were seeded onto the upper chamber. Matrigel and cells remaining in the upper chamber were removed after a 36 h incubation. The cells on the underside surface of the membrane were fixed using the Diff Quick Stain Kit (IMEB Inc., San Marcos, CA, USA) and the number of cells per field of view was counted. The value for each sample is the average number of cells from four different fields of view. All experiments were performed in triplicate and repeated three times.
Gelatin zymography
MMP-2 and MMP-9 enzymatic activities in the cell-culture medium were determined by SDS–PAGE gelatin zymography. Cells were seeded at 5 × 105 cells per 6-cm dish. Conditioned cell-culture medium from each dish was normalized to an equal amount of protein (100 μg), denatured in the sample buffer in the absence of reducing agents, and subjected to 10% SDS–PAGE containing 0.1% (w/v) gelatin (Acros Organics, Morris Plains, NJ, USA). The gel was incubated in the presence of 2.5% Triton X-100 at room temperature for 2 h, and then at 37 °C for 40 h in digestion buffer containing 1% Triton X-100, 10 mM CaCl2, 0.15 M NaCl and 50 mM Tris (pH 7.5). Gels were stained with Coomassie Blue R-250 and destained. Proteolysis was detected as a white band against a blue background.
References
Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 2001; 22: 153–183.
Kyriakis JM, Avruch J . Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 2001; 81: 807–869.
Uhlik MT, Abell AN, Cuevas BD, Nakamura K, Johnson GL . Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem Cell Biol 2004; 82: 658–663.
Johnson GL, Lapadat R . Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002; 298: 1911–1912.
Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ . Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol 1998; 8: 1049–1057.
Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ et al. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem 1995; 270: 7420–7426.
Lawler S, Fleming Y, Goedert M, Cohen P . Synergistic activation of SAPK1/JNK1 by two MAP kinase kinases in vitro. Curr Biol 1998; 8: 1387–1390.
Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ . MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 1996; 16: 1247–1255.
Gallo KA, Johnson GL . Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 2002; 3: 663–672.
Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ . Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol 2005; 25: 3670–3681.
Chadee DN, Kyriakis JM . MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol 2004; 6: 770–776.
Chen J, Miller EM, Gallo KA . MLK3 is critical for breast cancer cell migration and promotes a malignant phenotype in mammary epithelial cells. Oncogene 2010; 29: 4399–4411.
Bardelli A, Parsons DW, Silliman N, Ptak J, Szabo S, Saha S et al. Mutational analysis of the tyrosine kinome in colorectal cancers. Science 2003; 300: 949.
Shao RX, Kato N, Lin LJ, Muroyama R, Moriyama M, Ikenoue T et al. Absence of tyrosine kinase mutations in Japanese colorectal cancer patients. Oncogene 2007; 26: 2133–2135.
Soung YH, Lee JW, Kim SY, Nam SW, Park WS, Lee JY et al. Kinase domain mutation of MLK4 gene is uncommon in gastric and hepatocellular carcinomas. Dig Liver Dis 2006; 38: 283.
Kashuba VI, Grigorieva EV, Kvasha SM, Pavlova TV, Grigoriev V, Protopopov A . Cloning and initial functional characterization of Mlk4alpha and MLK4beta. Genomics Insights 2011; 4: 1–12.
Hanahan D, Weinberg RA . The hallmarks of cancer. Cell 2000; 100: 57–70.
Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994; 372: 739–746.
Shin I, Kim S, Song H, Kim HR, Moon A . H-Ras-specific activation of Rac-MKK3/6-p38 pathway: its critical role in invasion and migration of breast epithelial cells. J Biol Chem 2005; 280: 14675–14683.
Lezama R, Diaz-Tellez A, Ramos-Mandujano G, Oropeza L, Pasantes-Morales H . Epidermal growth factor receptor is a common element in the signaling pathways activated by cell volume changes in isosmotic, hyposmotic or hyperosmotic conditions. Neurochem Res 2005; 30: 1589–1597.
Chan PM, Lim L, Manser E . PAK is regulated by PI3K, PIX, CDC42, and PP2Calpha and mediates focal adhesion turnover in the hyperosmotic stress-induced p38 pathway. J Biol Chem 2008; 283: 24949–24961.
Cuenda A, Cohen P, Buee-Scherrer V, Goedert M . Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38). EMBO J 1997; 16: 295–305.
Nagase H, Woessner Jr JF . Matrix metalloproteinases. J Biol Chem 1999; 274: 21491–21494.
Schmalfeldt B, Prechtel D, Harting K, Spathe K, Rutke S, Konik E et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res 2001; 7: 2396–2404.
Seit-Nebi A, Cheng W, Xu H, Han J . MLK4 has negative effect on TLR4 signaling. Cell Mol Immunol 2012; 9: 27–33.
Swenson-Fields KI, Sandquist JC, Rossol-Allison J, Blat IC, Wennerberg K, Burridge K et al. MLK3 limits activated Galphaq signaling to Rho by binding to p63RhoGEF. Mol Cell 2008; 32: 43–56.
Du Y, Bock BC, Schachter KA, Chao M, Gallo KA . Cdc42 induces activation loop phosphorylation and membrane targeting of mixed lineage kinase 3. J Biol Chem 2005; 280: 42984–42993.
Acknowledgements
This work was supported by the National Institutes of Health Grant 1 R15 CA132006-01 grant (to DNC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Abi Saab, W., Brown, M. & Chadee, D. MLK4β functions as a negative regulator of MAPK signaling and cell invasion. Oncogenesis 1, e6 (2012). https://doi.org/10.1038/oncsis.2012.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/oncsis.2012.6
Keywords
This article is cited by
-
MLK4 promotes glucose metabolism in lung adenocarcinoma through CREB-mediated activation of phosphoenolpyruvate carboxykinase and is regulated by KLF5
Oncogenesis (2023)
-
MLK4 regulates DNA damage response and promotes triple-negative breast cancer chemoresistance
Cell Death & Disease (2021)
-
Upregulation of MLK4 promotes migratory and invasive potential of breast cancer cells
Oncogene (2019)
-
Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway
Tumor Biology (2015)