Abstract
Chronic inflammation is frequently associated with tumorigenesis in elderly people. By contrast, young people without chronic inflammation often develop tumors considered independent of chronic inflammation but driven instead by mutations. Thus, whether inflammation has a significant role in tumor progression in tumors driven by mutations remains largely unknown. Here we show that TNFα is required for the tumorigenesis of osteosarcoma, the most common tumor in children and adolescents. We show that transplantation of AX osteosarcoma cells, which harbor mutations driving c-Myc overexpression and Ink4a-deficiency, in wild-type mice promotes lethal tumorigenesis accompanied by ectopic bone formation and multiple metastases, phenotypes seen in osteosarcoma patients. Such tumorigenesis was completely abrogated in TNFα-deficient mice. AX cells have the capacity to undergo osteoblastic differentiation; however, that activity was significantly inhibited by TNFα treatment, suggesting that TNFα maintains AX cells in an undifferentiated state. TNFα inhibition of AX cell osteoblastic differentiation occurred through ERK activation, and a pharmacological TNFα inhibitor effectively inhibited both AX cell tumorigenesis and increased osteoblastic gene expression and increased survival of tumor-bearing mice. Lethal tumorigenesis of AX cells was also abrogated in IL-1α/IL-1β doubly deficient mice. We found that both TNFα and IL-1 maintained AX cells in an undifferentiated state via ERK activation. Thus, inflammatory cytokines are required to promote tumorigenesis even in mutation-induced tumors, and TNFα/IL-1 and ERK may represent therapeutic targets for osteosarcoma.
Similar content being viewed by others
Introduction
To date, one-third of the populations of developed countries die of malignant tumors.1 Continuous exposure to inflammatory cytokines is known to cause tumorigenesis;2 thus controlling chronic inflammation is crucial to prevent tumor progression, particularly in the elderly.3 By contrast, tumors developed due to mutations are thought to be largely driven by intrinsic signals emerging from damage to tumor-initiating genes, formation of chimeric proteins due to translocation or loss of tumor suppressor genes.4
Osteosarcoma is a rare malignancy but the most common bone sarcoma in children and adolescents.5 Osteosarcoma frequently metastasizes to tissues such as lung, leading to mortality.6 As osteosarcoma develops in young people free from chronic inflammation, tumor progression has been considered independent of inflammation. Osteosarcoma originates from mesenchymal stem cells and osteoblastic cells and is defined as an ‘osteoid-producing’ tumor.7 Thus, ectopic bone formation is frequently detected in osteosarcoma patients at both primary tumor sites and metastatic sites.8 As osteoid and bones form at the terminal stage of osteoblast differentiation, osteosarcoma exhibits terminally differentiated osteoblastic phenotypes. Osteosarcoma cells also exhibit differentiation-arrested phenotypes and continuous proliferation, as seen in other malignant tumors. How both differentiated and de-differentiated phenotypes are regulated concomitantly in osteosarcoma remains largely unknown. Osteosarcoma often promotes local inflammation and is thus considered an activator of local immune responses. Indeed, several inflammatory cytokines are reportedly upregulated in the sera of osteosarcoma patients;9 thus far, however, the roles of inflammation in osteosarcoma have not been characterized.
Development of protocols employing cytotoxic chemotherapy drugs as well as diagnostic tools such as magnetic resonance imaging have improved the prognosis and survival rate for osteosarcoma patients. Nonetheless, ∼30% of osteosarcoma patients die with metastasis or tumor recurrence,10 and the survival rate of osteosarcoma patients has not substantially improved in the last 20 years.11 Therefore, novel targets are required to treat these patients.
Animal models are useful for the development of new chemotherapeutic agents for osteosarcoma. Historically, a spontaneous osteosarcoma animal model or a model based on exposure to a radioactive agent has been utilized.12 However, ectopic bone formation is not evident in the spontaneous model, and only a small proportion of human osteosarcomas are radiation-induced.13 Xenograft models using tissues from osteosarocoma patients, such as MG63 cells, have also been developed but, again, ectopic bone formation has not been detected in these animals. More recently, a transplantable mouse osteosarcoma model has been developed based on the AX cell line.14 Mesenchymal AX cells isolated from INK4a-deficient mice were transduced with c-Myc and transplanted into wild-type mice, resulting in the formation of osteosarcoma-producing osteoid and ectopic bone.14 In wild-type mice the frequency of tumor formation and metastasis to various tissues is reportedly 100% in this model, making it a useful tool to analyze mechanisms of tumor development in a de-differentiated state accompanied by ectopic bone formation, as seen in human osteosarcoma patients.
Here we found that TNFα produced by host macrophages functions to maintain osteoarcoma cells in an undifferentiated state and is required for tumor progression. TNFα-deficient mice transplanted with AX cells exhibited completely abrogated tumor development, and pharmacological inhibition of TNFα inhibited tumor growth and elevated osteoblastic differentiation in vivo. Similarly, osteoblastogenesis in AX cells was significantly inhibited by IL-1 treatment, and tumor development was abrogated in IL-1α/IL-1β doubly deficient mice. Finally, we show that TNFα and IL-1 inhibited osteoblastic differentiation in AX cells through ERK activation. Thus, exogenous inflammatory cytokines are required for tumorigenesis by maintaining an undifferentiated state even in mutation-induced osteosarcoma. These findings suggest that inflammatory factors and ERKs represent potential therapeutic targets for osteosarcoma.
Results
IL-6 is upregulated in osteosarcoma-bearing mice but does not function in osteosarcoma progression
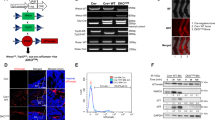
We utilized the AX osteosarcoma model to establish osteosarcoma in vivo and analyze levels of serum cytokines and chemokines in tumor-bearing mice. Among cytokines and chemokines tested, we observed significantly high levels of IL-6, an inflammatory cytokine, in AX cell-bearing mice compared with non-tumor-bearing controls (Figure 1a). Inflammation is often seen in human osteosarcoma patients,9 and IL-6 is implicated in the development of various tumors.15, 16, 17 Thus, we transplanted AX cells into IL-6-deficient or wild-type mice in order to compare mouse survival rates (Figure 1b). However, survival rates of AX cell-bearing mice of either genotype were comparable (Figure 1b), suggesting that IL-6 does not function in AX cell tumorigenesis in vivo.
Serum IL-6 levels are upregulated in mice transplanted with AX cells, but IL-6 does not function in lethal AX cell progression. (a) AX cells were transplanted into wild-type mice intraperitoneally, and 2 weeks later serum was collected and various cytokine/chemokine levels were measured and compared with those in non-tumor-bearing mice. Data are shown as mean relative cytokine and chemokine levels in serum from AX cell-injected mice compared with those from non-tumor-bearing mice (n=5). (b) IL-6−/− mice and littermates were transplanted with AX cells intraperitoneally and their survival curves were drawn (n=5).
TNFα produced by macrophages is required for osteosarcoma progression
Next, we analyzed signals upstream of IL-6 induction in AX cells, and found that IL-6 mRNA level was significantly upregulated by TNFα stimulation of AX cells (Figure 2a). To assess which cells express TNFα in vivo, AX cells, tumor-associated CD11b-positive macrophages and CD3-positive T cells were isolated from primary tumor sites in tumor-bearing wild-type mice, and RT–PCR analysis for TNFα was performed. This analysis indicated that TNFα was produced by macrophages (Figure 2b). Immunohistochemical analysis confirmed TNFα expression in CD11b-positive tumor-associated macrophages (Figure 2b). When we transplanted AX cells into TNFα-deficient or wild-type mice, we found that in mice lacking TNFα, lethal tumor progression was completely abrogated in vivo (Figure 2c). The bone marrow (BM) of TNFα-deficient mouse was reconstructed by transplantation using wild-type or TNFα-deficient BM cells, followed by lethal irradiation, and AX cells were then transplanted into both types of mice (Figure 2d). TNFα-deficient mice transplanted with TNFα-deficient BM cells survived, whereas lethal tumor progression was seen in all mice transplanted with wild-type BM cells (Figure 2d). These data suggest that local TNFα production by macrophages is required for AX cell tumor progression, despite the fact that AX cells harbor mutations that lead to tumor development. Similarly, IL-6 expression was significantly upregulated by IL-1β stimulation in AX cells in vitro (Supplementary Figure S1a), and lethal tumor progression in IL-1α and IL-1β doubly deficient mice (IL-1 DKO) transplanted with AX cells was totally abrogated compared with wild-type mice (Supplementary Figure S1b). Thus, TNFα and IL-1 promote tumor progression, which underlies mortality.
TNFα produced by tumor-associated macrophages is essential for AX cell lethality. (a) Total RNA was prepared from AX cells treated with TNFα (10 ng/ml) for 24 h, and IL-6 expression relative to β-actin was analyzed by quantitative real-time PCR (n=3).*P<0.01. (b) Wild-type mice were transplanted with AX cells intraperitoneally. Seven days later, AX cells (EGFP+ cells), CD3+ T cells and CD11b+ macrophages were sorted, and TNFα expression was analyzed by RT–PCR (left). TNFα protein expression was also analyzed by immunofluorescence staining at primary tumor sites. Paraffin sections were stained with PE-conjugated rat anti-CD11b antibody and rabbit anti-TNFα antibody followed by Alexa488-conjugated anti-rabbit Ig’ antibody and observed under a fluorescent microscopy (right). Nuclei were visualized using DAPI. Bar, 100 μm. (c) TNFα−/− mice and wild-type littermates were transplanted with AX cells intraperitoneally and survival curves were drawn (n=5). (d) TNFα−/−mice were lethally irradiated and BM cells were reconstituted by transplantation of BM cells isolated from wild-type or TNFα−/− mice. Four months later, AX cells were transplanted into mice intraperitoneally and survival curves were drawn (n=5).
Blocking TNFα by soluble TNFα receptor inhibits lethal tumor progression
Next, we examined the effects of pharmacological TNFα-ablation on tumor progression in vivo (Figure 3). Etanercept, a decoy TNFα receptor, is a soluble form of TNFα receptor and is a TNFα inhibitor utilized to treat patients with rheumatoid arthritis by subcutaneous injection.18 The significantly upregulated IL-6 expression seen in AX cells following TNFα stimulation was inhibited by TNFα inhibitor treatment in vitro (Figure 3a). We then injected TNFα inhibitor subcutaneously into AX cell-bearing wild-type mice and observed that treatment significantly increased the survival of tumor-bearing mice relative to vehicle-treated mice (Figure 3b). Thus, TNFα could serve as a target to antagonize the lethal progression of osteosarcoma.
Etanercept improves the survival rate of AX cell-transplanted mice in vivo. (a) Total RNA was prepared from AX cells treated with TNFα (10 ng/ml) and Etanercept (Eta) (0.25 ng/ml, 2.5 ng/ml) for 24 h, and IL-6 expression relative to β-actin was analyzed by quantitative real-time PCR. Data represent mean IL-6 expression relative to β-actin±s.d. (n=3). *P<0.01. (b) Wild-type mice injected with AX cells intraperitoneally at day 0 were treated with Etanercept or vehicle for 3 weeks (twice per week) from day 0, and survival curves were drawn (n=5).
TNFα inhibits osteoblastic differentiation of AX cells
TNFα promotes apoptosis by activating caspase 3.19 Indeed, levels of cleaved caspase 3, the activated form of caspase 3, increased following TNFα stimulation of primary mouse embryonic fibroblasts in vitro (Figure 4a). In contrast, cleaved caspase 3 levels were not elevated by TNFα stimulation of AX cells (Figure 4a), suggesting that AX cells are resistant to TNFα-induced apoptosis. AX cell proliferation in vitro was unchanged in the presence of TNFα, TNFα inhibitor or both (Figure 4b). However, the expression of osteoblastic genes, such as alkaline phosphatase (ALP), osteocalcin (Oc) and Runx2, was significantly downregulated in AX cells following TNFα treatment in vitro (Figure 4c), as were ALP protein levels (Figure 4d). Similar to TNFα, IL-1β treatment also significantly inhibited ALP, Oc and Runx2 expression in AX cells (Supplementary Figure S2). Furthermore, ALP, Oc and runx2 expression in AX cells in TNFα inhibitor-treated or IL-1 DKO mice was significantly upregulated compared with AX cells in vehicle-treated mice in vivo (Figure 4e and Supplementary Figure S3), suggesting that TNFα and IL-1 inhibit osteoblastic differentiation and maintain osteosarcoma cells in an undifferentiated state.
TNFα inhibits osteoblastic differentiation of AX cells. (a) Whole-cell lysates of mouse embryonic fibroblasts or AX cells stimulated with TNFα (10 ng/ml) or Staurosporine (10 μg/ml) were analyzed by immunoblotting to detect cleaved caspase 3 or caspase 3. Actin served as an internal control. (b) Proliferation of AX cells stimulated with TNFα (10 ng/ml), Etanercept (Eta) (2.5 ng/ml) or both was analyzed. (c) Total RNA was prepared from AX cells treated with or without TNFα (10ng/ml) for 24 h, and expression of ALP, Osteocalcin (Oc) or Runx2 relative to β-actin was analyzed by quantitative real-time PCR. Data represent mean ALP, Oc or Runx2 expression relative to β-actin±s.d. (n=3).*P<0.01;**P<0.05. (d) ALP activity of AX cells treated with or without TNFα (10 ng/ml) for 24 h was analyzed (n=3). **P<0.05. (e) AX cells were transplanted into wild-type mice, and mice were treated with Etanercept or vehicle. After 10 days, AX cells were sorted from primary tumor sites, total RNA was prepared and expression of ALP, Oc or Runx2 relative to β-actin was analyzed by quantitative real-time PCR (n=3).**P<0.05.
TNFα inhibits osteoblastic differentiation via the ERK pathway in AX cells
The NFκB pathway is the major signaling cascade downstream of TNFα; however, NFκB inhibition did not rescue inhibited osteoblastic differentiation mediated by TNFα seen in AX cells (Figure 5a). Similarly, inhibition of p38 and JNK did not rescue inhibited osteoblastic differentiation mediated by TNFα in AX cells (Figure 5a). However, ERK inhibition by a MEK inhibitor U0126 effectively rescued inhibited osteoblastic differentiation seen in AX cells following TNFα upregulation, and expression of osteoblastic genes, such as ALP, Oc and Runx2, which had been inhibited by TNFα, was restored by a treatment with an ERK inhibitor in vitro (Figure 5a). Neither TNFα treatment, IL-1β treatment nor ERK inhibition altered AX cell proliferation (Figure 5b), suggesting that ERK is specifically required to inhibit osteoblastic differentiation rather than to activate AX cell proliferation. These results suggest that TNFα and IL-1 promote tumorigenesis by maintaining AX cells in an undifferentiated state via ERK activation.
Inhibition of osteoblastogenesis of AX cells by TNFα is mediated by ERK. (a) Total RNA was prepared from AX cells treated with TNFα (10 ng/ml) in the presence or absence of the inhibitors of JNK, p38, ERK or NFκB, and expression of ALP, Osteocalcin (Oc) or Runx2 relative to β-actin was analyzed by quantitative real-time PCR. Data represent mean ALP, Oc or Runx2 expression relative to β-actin±s.d. (n=3). **P<0.05; NS, not significant. (b) Proliferation of AX cells stimulated with TNFα, IL-1β, TNFα plus an ERK inhibitor or IL-1β plus an ERK inhibitor for 24 h was analyzed (n=3).
Discussion
Numerous factors have been implicated in tumorigenesis, such as mutations, chronic inflammation resulting from bacterial or viral infection and prolonged exposure to radiation or oncogenic chemicals.20 Tumors associated with mutations, chromosomal translocations or mutations in tumor suppressor genes such as breast cancer susceptibility genes 1 and 2 undergo rapid tumorigenesis,21 and such tumors are generally considered not to require inflammatory stimuli. However, our results in an osteosarcoma model indicate that inflammation is required for tumorigenesis even in mutation-induced tumors.
AX cells are tumor cells marked by INK4a deficiency and c-Myc oncogene overexpression.14, 22, 23, 24, 25 FGF2 produced by tumor-associating fibroblasts reportedly contributes to maintain cellular immaturity and aggressiveness.23 Interestingly, we found that inhibition of osteoblastic differentiation by TNFα or IL-1 through ERK was required for AX cell tumorigenesis through the maintenance of an undifferentiated state. Although IL-6 is an inflammatory cytokine implicated in tumorigenesis of various cancers,15, 16 IL-6 was not required for tumor development in this osteosarcoma model. Therefore, inflammatory cytokines, such as TNFα or IL-1 or ERKs could serve as therapeutic targets for such mutation-induced tumors.
Osteosarcoma is an osteoid-producing tumor, and given that osteoid and bone matrix are produced at the terminal stage of osteoblast differentiation, osteosarcoma exhibits terminally differentiated osteoblastic phenotypes. Osteosarcoma cells reportedly express bone morphogenic protein and form ectopic bone.26 Nonetheless, maintenance of an undifferentiated state occurs in osteosarcoma, even under the highly differentiated condition evidenced by ectopic tumor bone formation or bone morphogenic protein expression.
Proliferation is terminated by differentiation signals in normal cells. In tumors, differentiation is disrupted or severely arrested, allowing tumors to continuously proliferate and promoting tumor progression. Thus, differentiation-inducing therapy is often effective in differentiation-arrested malignant tumors such as acute promyelocytic leukemia (PML).27 Most PML occurs in children, driven by chromosomal translocation between chromosomes 15 and 17, which gives rise to the chimeric protein PML-RARα and induces differentiation arrest.28 Treatment of PML patients with all-trans retinoic acid promotes PML cell differentiation and significantly increases survival rate.27 In our model, differentiation arrest in osteosarcoma occurs via the TNFα/IL-1-ERK pathway. Our findings may also apply to other tumor types and contribute to the development of differentiation-inducing therapies in those malignancies. ERK signaling has diverse roles in regulating cellular proliferation and differentiation.29 Various cytokine and growth factor signals stimulate the ERK pathway, and subsequent ERK phosphorylation transduces cellular responses to that stimulation.30 Although ERK signaling has been implicated in inducing cellular differentiation,31 our model demonstrates that ERK induces differentiation arrest in osteosarcoma, suggesting that responses to ERK signals are likely cell type-specific. Assessing inflammation or ERK activation in tumor biopsy samples before starting chemotherapies might implicate inflammatory cytokines or ERK as additional or alternative therapeutic targets for tumors, in addition to conventional cytotoxic chemotherapies.
Overall, our findings shed light on novel mechanisms of tumorigenesis in mutation-induced tumors and suggest a novel differentiation-inducing therapy to treat those tumors by targeting ERK and inflammatory cytokines such as TNFα and IL-1.
Materials and methods
Chemicals and reagents
Etanercept, a tumor necrosis factor antagonist, was purchased from Takeda Pharmaceutical Co. (Osaka, Japan). AZD6244, a MEK1 inhibitor, was purchased from Selleck Chemicals (Houston, TX, USA). Recombinant mouse IL-1β and mouse TNFα were purchased from PeproTech Ltd. (London, UK).
Cell culture and real-time PCR analysis
AX cells were established and characterized by Shimizu et al.,14, 22, 23, 24 and were maintained in DMEM (Sigma-Aldrich, St Louis, MO, USA) containing 10% FBS (JRH Biosciences, Kansas, TX, USA), 1% GlutaMax and antibiotics.
Total RNAs were isolated from either cultured or sorted cells using TRIzol reagent (Invitrogen, Tokyo, Japan). cDNAs were synthesized from total RNAs using oligo(dT) primer and reverse transcription (Wako Pure Chemicals Industries, Osaka, Japan). Real-time PCR was performed using SYBR Premix ExTaq II (Takara Bio Inc., Shiga, Japan) with a DICE Thermal cycler (Takara Bio Inc.), according to the manufacturer’s instructions. Samples were matched to a standard curve generated by amplifying serially diluted products using the same PCR reactions. β-actin expression served as an internal control. Primer sequences were as follows:
β-actin forward: 5′-TGAGAGGGAAATCGTGCGTGAC-3′;
β-actin reverse: 5′-AAGAAGGAAGGCTGGAAAAGAG-3′;
IL6 forward: 5′-CAAAGCCAGAGTCCTTCAGAG-3′;
IL6 reverse: 5′-GTCCTTAGCCACTCCTTCTG-3′;
IL1alpha forward: 5′-TGCAGTCCATAACCCATGATC-3′;
IL1alpha reverse: 5′-ACAAACTTCTGCCTGACGAG-3′;
TNF alpha forward: 5′-CTTCTGTCTACTGAACTTCGGG-3′;
TNF alpha reverse: 5′-CAGGCTTGTCACTCGAATTTTG-3′;
ALP forward: 5′-ACACCTTGACTGTGGTTACTGCTGA-3′;
ALP reverse: 5′-CCTTGTAGCCAGGCCCGTTA-3′;
Osteocalcin forward: 5′-TAGCAGACACCATGAGGACCCT-3′;
Osteocalcin reverse: 5′-TGGACATGAAGGCTTTGTCAGA-3′;
Runx2 forward: 5′-GACGTGCCCAGGCGTATTTC-3′;
Runx2 reverse: 5′-AAGGTGGCTGGGTAGTGCATTC-3′.
Immunoblotting analysis
Whole-cell lysates were prepared from BM cultures using RIPA buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 10 mM Tris–HCl (pH 7.5), 5 mM EDTA and a protease inhibitor cocktail; Sigma-Aldrich). Equivalent amounts of protein were separated by SDS–PAGE and transferred to a PVDF membrane (Millipore, Billerica, MA, USA). Proteins were detected using the following antibodies: anti-pERK (#9106; Cell Signaling Technology, Inc., Beverly, MA, USA), anti-ERK (#9107; Cell Signaling Technology, Inc.), cleaved caspase 3 (#9661; Cell Signaling Technology, Inc.), caspase 3 (#9665; Cell Signaling Technology, Inc.) and anti-actin (A2066; Sigma-Aldrich).
Histopathology and fluorescent immunohistochemistry
Mice were killed and the primary tumor was fixed in 10% neutral-buffered formalin, decalcified in 10% ethylenediaminetetraacetic acid (EDTA) (pH 7.4), embedded in paraffin and then cut into 4-μm sections. For each fluorescent immunohistochemistry assay, sections of 4 μm thickness were cut and subjected to microwave treatment for 5 min in 1 mM EDTA (pH 8.0) for antigen retrieval. After blocking with 0.1% BSA in 100 mM Tris–HCl (pH 7.6), 150 mM NaCl, 0.01% Tween-20 (TBST) for 20 min, sections were incubated for 1 h with rabbit anti-mouse GFP, goat anti-mouse TNFα (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) diluted 1:100 and rat anti-mouse FITC-CD11b (BD Biosciences, CA, USA) diluted 1:200. After washing with TBST, sections were incubated with Alexa Fluor 488-conjugated donkey anti-goat IgG (Invitrogen) diluted 1:200 and Alexa Fluor 546-conjugated rabbit anti-mouse IgG (Invitrogen) diluted 1:200. Finally, sections were mounted using Dako fluorescence mounting medium. Nuclei were stained with TOTO3 (1:750; Invitrogen). Images were acquired with a laser confocal microscope (FV1000-D, Olympus, Tokyo, Japan).
Cell proliferation assay
Cells were transferred to 96-well tissue culture plates and cultured under indicated conditions. Cell proliferation was measured using a Cell Counting kit-8 (Dojindo Molecular Technologies, Inc. Kumamoto, Japan). The OD at 450 nm was read on a Labsystems Multiscan MS (Analytical Instruments, LLC, Golden Valley, MN, USA).
Alkaline phosphatase activity assay
Cells were transferred to 96-well tissue culture plates and cultured under indicated condition. Alkaline phosphatase activity was measured by TRAP and ALP Assay Kit (Takara Bio Inc.). The OD at 405 nm was read on a Labsystems Multiscan MS (Analytical Instruments).
Animal studies
TNFα−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). IL-1α−/−IL-1β−/− mice were provided by Professor Iwakura (Tokyo University of Science). Expression of various cytokines in mouse serum was evaluated using Milliplex MAP Cytokine/Chemokine kit (Millipore). All animals were maintained under specific pathogen-free conditions in animal facilities certified by the Keio University School of Medicine Animal Care Committee. Animal protocols were approved by that committee. A total of 2.5 × 106 AX cells were injected into mice intraperitoneally or subcutaneously and survival curves were drawn. Alternatively, subcutaneous tumors were analyzed 10 days after AX cell injection. Some mice were treated with Etanercept (5 mg/kg, twice a week, intraperitoneally) or vehicle buffer, and survival curves were drawn or subcutaneous tumors were analyzed 10 days after cell injection. Etanercept was diluted in 100 μl PBS.
Statistical analysis
Statistical analysis was performed using Student’s t-test or one-way ANOVA, followed by a Tukey–Kramer test to determine significance between groups. In this context, significant differences were defined as P<0.05.
References
World Health Organization Ten Statistical Highlights in Global Public Health. World Health Statistics. World Health Organization, Geneva, 2007.
Vendramini-Costa DB, Carvalho JE . Molecular link mechanisms between inflammation and cancer. Curr Pharm Des 2012; 18: 3831–3852.
Vasto S, Carruba G, Lio D, Colonna-Romano G, Di Bona D, Candore G et al. Inflammation, ageing and cancer. Mech Ageing Dev 2009; 130: 40–45.
Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 2010; 466: 869–873.
Ottaviani G, Jaffe N . The epidemiology of osteosarcoma. Cancer Treat Res 2009; 152: 3–13.
Nakamura T, Matsumine A, Matsubara T, Asamuma K, Niimi R, Uchida A et al. Retrospective analysis of metastatic sarcoma patients. Oncol Lett 2011; 2: 315–318.
Dahlin DC . Pathology of osteosarcoma. Clin Orthop Relat Res 1975; 111: 23–32.
Kim SJ, Choi JA, Lee SH, Choi JY, Hong SH, Chung HW et al. Imaging findings of extrapulmonary metastases of osteosarcoma. Clin Imaging 2004; 28: 291–300.
Rutkowski P, Kamińska J, Kowalska M, Ruka W, Steffen J . Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: correlations with local tumor extent and prognosis. J Surg Oncol 2003; 84: 151–159.
Picci P . Osteosarcoma (osteogenic sarcoma). Orphanet J Rare Dis 2007; 23: 2–6.
Mirabello L, Troisi RJ, Savage SA . Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009; 115: 1531–1543.
Bensted JPM, Blackett NM, Lamerton LF . Histological and dosimetric considerations of bone tumour production with radioactive phosphorus. Br J Radiol 1961; 34: 160–175.
Sheppard DG, Libshitz HI . Post-radiation sarcomas: a review of the clinical and imaging features in 63 cases. Clin Radiol 2001; 56: 22–29.
Shimizu T, Ishikawa T, Sugihara E, Kuninaka S, Miyamoto T, Mabuchi Y et al. c-MYC overexpression with loss of Ink4a/Arf transforms bone marrow stromal cells into osteosarcoma accompanied by loss of adipogenesis. Oncogene 2010; 29: 5687–5699.
Waldner MJ, Foersch S, Neurath MF . Interleukin-6–a key regulator of colorectal cancer development. Int J Biol Sci 2012; 8: 1248–1253.
Hoejberg L, Bastholt L, Schmidt H . Interleukin-6 and melanoma. Melanoma Res 2012; 22: 327–333.
Sansone P, Bromberg J . Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol 2012; 30: 1005–1014.
Feldmann M, Maini RN . Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med 2003; 9: 1245–1250.
Paroni G, Henderson C, Schneider C, Brancolini C . Caspase-2-induced apoptosis is dependent on caspase-9, but its processing during UV- or tumor necrosis factor-dependent cell death requires caspase-3. J Biol Chem 2001; 276: 21907–21915.
Morrison WB . Inflammation and cancer: a comparative view. J Vet Intern Med 2012; 26: 18–31.
Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst 2013; 105: 812–822.
Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 2009; 206: 1089–1102.
Shimizu T, Ishikawa T, Iwai S, Ueki A, Sugihara E, Onishi N et al. Fibroblast growth factor-2 (Fgf2) is an important factor that maintains cellular immaturity and contributes to aggressiveness of osteosarcoma. Mol Cancer Res 2012; 10: 454–468.
Ishikawa T, Shimizu T, Ueki A, Yamaguchi SI, Onishi N, Sugihara E et al. Twist2 functions as a tumor suppressor in murine osteosarcoma cells. Cancer Sci 2013; 104: 880–888.
Ueki A, Shimizu T, Masuda K, Yamaguchi SI, Ishikawa T, Sugihara E et al. Up-regulation of Imp3 confers in vivo tumorigenicity on murine osteosarcoma cells. PLoS One 2012; 7: e50621.
Yoshikawa K, Takaoka K, Hamada H, Ono K . Clinical significance of bone morphogenetic activity in osteosarcoma. A study of 20 cases. Cancer 1985; 56: 1682–1687.
Warrell RP Jr, Frankel SR, Miller WH Jr, Scheinberg DA, Itri LM, Hittelman WN et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin (all-trans retinoic acid). N Engl J Med 1991; 324: 1585.
Kakizuka A, Miller WH Jr, Umesono K, Warrell RP Jr, Frankel SR, Murty VV et al. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell 1991; 66: 663–674.
Peyssonnaux C, Eychène A . The Raf/MEK/ERK pathway: new concepts of activation. Biol Cell 2001; 93: 53–62.
Hagemann C, Blank JL . The ups and downs of MEK kinase interactions. Cell Signal 2001; 13: 863–875.
Marshall CJ . Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 1995; 80: 179–185.
Acknowledgements
T Miyamoto was supported by a Grant-in-aid for Scientific Research, Takeda Science Foundation, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
This work is licensed under a Creative Commons Attribution 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/
About this article
Cite this article
Mori, T., Sato, Y., Miyamoto, K. et al. TNFα promotes osteosarcoma progression by maintaining tumor cells in an undifferentiated state. Oncogene 33, 4236–4241 (2014). https://doi.org/10.1038/onc.2013.545
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2013.545
Keywords
This article is cited by
-
The IL-17-IL-17RA axis is required to promote osteosarcoma progression in mice
Scientific Reports (2023)
-
Achaete-scute complex-like 2 regulated inflammatory mechanism through Toll-like receptor 4 activating in stomach adenocarcinoma
World Journal of Surgical Oncology (2022)
-
Osteonecrosis development by tooth extraction in zoledronate treated mice is inhibited by active vitamin D analogues, anti-inflammatory agents or antibiotics
Scientific Reports (2022)
-
Tooth extraction in mice administered zoledronate increases inflammatory cytokine levels and promotes osteonecrosis of the jaw
Journal of Bone and Mineral Metabolism (2021)
-
The smac mimetic LCL161 targets established pulmonary osteosarcoma metastases in mice
Clinical & Experimental Metastasis (2021)