Abstract
Primary cilia can act as either a negative or positive regulator of the hedgehog (Hh) signaling pathway. Many cartilage tumors are characterized by abnormal activation of the Hh pathway. Here, we report that the presence of primary cilia occurs at a low frequency (12.4%) in neoplastic chondrocytes from malignant human chondrosarcomas, compared with chondrocytes from normal articular cartilage (67.7%). To determine the function of primary cilia in cartilaginous neoplasia, we studied benign cartilage tumors that are formed in mice by chondrocyte-specific overexpression of Gli2, a downstream transcriptional activator of the Hh pathway. Col2A1-Gli2 mice were crossed with Ift88+/− mice, which display a partial loss of ciliogenesis. Surprisingly, cartilage tumors developed in Ift88+/− mice that were phenotypically similar to those that arise in Col2A1-Gli2 mice. Further activation of the Hh pathway was observed in Col2A1-Gli2; Ift88+/− mice compared with either Col2A1-Gli2 or Ift88+/− mice, which was associated with an increased incidence of cartilage tumors. Chondrosarcomas were established in explant cultures, and treated with choral hydrate, which disrupts the functional primary cilia. Thus, treatment resulted in hyperactivity of the Hh signaling pathway, as well as cellular changes that could promote tumor growth. Primary cilia functions to inhibit Hh signaling in neoplastic chondrocytes. The activation of Hh signaling is sufficient to induce benign cartilage tumors without another oncogenic initiating event. Moreover, as primary cilia suppress Hh pathway activation in chondrosarcoma, cellular mechanisms inhibiting proper cilia function may be important in maintaining the neoplastic phenotype.
Similar content being viewed by others
Introduction
Chondrosarcoma is a tumor composed of neoplastic chondrocytes originating in bone that can progress from pre-existing benign tumors, such as enchondromas. During skeletal development, chondrocytes undergo highly controlled stages of differentiation in the growth plate, eventually becoming replaced by bone. Enchondromas can occur as a result of abnormal regulation of chondrocyte differentiation and the persistence of growth plate tissue in mature bones. Formation of enchondroma-like lesions can be induced in transgenic mice by chondrocyte-specific expression of the hedgehog (Hh) transcriptional activator, Gli2. Most cartilage tumors demonstrate activation of the Hh pathway at a similar level to that of growth plate tissue.1 However, mutations of Hh pathway components are infrequent in chondrosarcoma.2
The Hh pathway becomes activated upon binding of the Hh ligand to its receptor patched (Ptch) to relieve its constitutive inhibition on the Smo transmembrane protein. Subsequent downstream signaling events lead to the activation of Gli transcription factors to regulate target gene expression. In vertebrates, the role of primary cilia has been demonstrated to be important for proper Hh signaling in various tissues. These sensory organelles protrude from the cell surface to detect changes in the extracellular environment, which is translated into specific responses of the intraflagellar transport system. Therefore, cilia are believed to act as specialized signaling centers for Hh pathway activity,3 through bidirectional movement of proteins between the base and tip of the cilium. Numerous human disorders attributed to the disruption of ciliogenesis display developmental defects characteristic of altered Hh signaling, such as polydactyly in patients with Bardet–Biedl syndrome and Meckel syndrome.4
Primary cilia of growth plate chondrocytes are oriented along the longitudinal axis of bone at proliferative and hypertrophic stages of differentiation.5 By establishing chondrocyte polarity, it is proposed that primary cilia facilitate the columnar organization of proliferative chondrocytes and controlled longitudinal growth of the developing bone. Disturbance of the intraflagellar transport system in mice leads to improper ciliogenesis and defects in the columnar orientation of growth plate chondrocytes.6, 7, 8 This is supported by the random orientation of primary cilia observed in osteochondroma; a common benign bone tumor with similar histological features to the growth plate, apart from an organized pattern of cell growth. Similarly, primary cilia of articular chondrocytes have a clear orientation away from the articulating surface, while osteoarthritic cells form abnormal cell clusters with primary cilia oriented towards the center.9 Aberrant activation of the Hh pathway is detected in osteoarthritic cartilage.10 Furthermore, transgenic mouse studies show that the absence of primary cilia activates Hh signaling and causes an osteoarthritis like phenotype in articular chondrocytes.11
Several types of cancers, in which Hh signaling is activated, such as renal, brain, pancreatic and breast cancer are reported to have a decrease in the incidence of primary cilia compared with normal surrounding tissues.12, 13, 14, 15, 16, 17 However, whether the cilium in Hh-dependent tumorigenesis can function as an activator or repressor is dependent on the oncogenic initiating event. For instance, in the presence of Gli2 activation, loss of cilia results in a more severe tumor phenotype in basal cell carcinoma.16 Furthermore, constitutively active Gli2 expression can cause medulloblastoma (MB) in mice, but only in the absence of cilia.12 Conversely, when basal cell carcinoma and MB are induced by overexpression of a constitutively active form of Smo (SmoM2), removal of cilia has a suppressive effect on tumorigenesis. Interestingly, MB do not form in the absence cilia, indicating that the primary cilium is required for SmoM2-induced oncogenesis. Thus, there is a variation in the response to ciliary loss between tumor types. Here, we investigated the role of the primary cilia in cartilage neoplasia.
Results
Most chondrocytes from chondrosarcomas and enchondromas lack primary cilia
Human chondrosarcomas were double stained for acetylated-α-tubulin and γ-tubulin to observe the presence of cilia within these tumors. Human articular cartilage samples were used for comparative controls. Primary cilia were identified by morphological characteristics, positive acetylated-α-tubulin staining, as well as adjacent localization to the microtubule organizing centers using a γ-tubulin antibody. Analysis of tumor cells was performed using confocal microscopy that allowed for a complete 3-dimensional view of each cell (Supplementary Figure 1). Cells within chondrosarcomas displayed the presence of cilia at a low frequency (12.4±7.1%) compared with human articular samples (63.9±8.6%, Figures 1a and b). Variability in ciliary incidence was not found to be associated with either a particular subtype of chondrosarcoma or histological grade. Overall, the frequency of cells lacking primary cilia ranged from 70–100% of tumor cells. As ciliary assembly is associated with interphase and cell cycle arrest, chondrosarcoma samples were stained with proliferative markers (proliferating cell nuclear antigen (PCNA) and Ki67) to determine whether the absence of cilia reflected a highly proliferative state of these tumors. Proliferative cells ranged from 15–31% of the total number of chondrosarcoma cells analyzed, suggesting that proliferative cells alone could not account for the low frequency of primary cilia observed. Furthermore, there is no correlation between the frequency of cilia and the number proliferative cells in individual chondrosarcoma samples (R2=0.2488). The absence of a relationship between these two factors was confirmed using Spearman’s rank correlation (rs=−0.4233).
Cilia are present in cartilage tumors at a low frequency. Human chondrosarcomas and enchondromas were analyzed using antiacetylated-α-tubulin (red) and anti-γ-tubulin (green) antibodies to identify primary cilia and microtubule organizing centers, respectively. Nuclei are stained with 4',6-diamidino-2-phenylindole (DAPI) (blue). All tumors were analyzed using a confocal microscope for Z-stacking of each section; confirming the presence/absence of primary cilia. (a) Representative samples of chondrosarcoma and enchondroma showing sparse numbers of cells displaying primary cilia. Conversely, normal human articular cartilage samples display the presence of primary cilia in the majority of cells. Identified primary cilia are indicated by white arrowheads. Magnification is shown at × 25 in the first row and × 63 in the second row, with scale bars as 18 and 7 μm in length, respectively. (b) Comparison of the proportion of cells exhibiting primary cilia in chondrosarcoma, enchondroma and normal cartilage. Error bars represent 95% confidence intervals, with the asterisk indicating a statistical significance of P<0.05.
As precursor lesions of chondrosarcomas, human enchondromas were also analyzed for the presence of primary cilia to elucidate whether ciliary defects are a common feature of both benign and malignant cartilage tumors. Similar to that of malignant chondrosarcoma, 13.4±2.6% of tumor cells were observed to display primary cilia (Figure 1b). The similar incidences of cilia in benign and malignant cartilage tumors suggest that the loss of cilia is not responsible for the progression from enchondromas to chondrosarcoma.
Loss of primary cilia in chondrosarcomas results in enhanced Hh pathway activation
To determine the role of the primary cilia in regulating the Hh pathway within the neoplastic chondrocytes, human chondrosarcoma explants were treated with chloral hydrate to ablate cilia by causing a breakdown at the junction of the cilium and the basal body. The successful removal of cilia was confirmed using immunofluorescence (Figure 2a). The samples were subsequently treated with Hh modulators to observe changes in pathway activity. Treatment with chloral hydrate alone did not alter the expression of target genes, PTCH1 and GLI1. However, activation of the pathway was enhanced in chloral hydrate treated samples in response to Hh ligand, compared with untreated controls. Conversely, treatment with cyclopamine did not inhibit the Hh pathway in chloral hydrate treated explants (Figure 2b). Immunohistochemical analysis of chondrosarcoma explants revealed that the Hh pathway activation is also associated with increases in proliferation and reduction in apoptosis (Figure 2c). Treatment of explants with Shh ligand resulted in a 1.5-fold increase in proliferation that was further elevated to a greater than twofold increase in samples lacking primary cilia. Ligand-dependent Hh activation also inhibited apoptosis at a 2.5-fold reduction that was further reduced to fourfold in cilia-deficient samples. Interestingly, cyclopamine treatment was unable to effectively rescue the effect of Hh stimulation on proliferation and apoptosis in chloral hydrate treated explants. Thus, cilia in neoplastic chondrocytes will attenuate Hh signaling, and are required for the inhibitory effects of cyclopamine.
Primary cilia suppresses Hh pathway activity in human chondrosarcomas. Human chondrosarcoma explants were treated with chloral hydrate to chemically remove primary cilia from the cells. Explants were then harvested to observe changes in Hh pathway activity in the presence or absence of functional cilia. (a) Immunofluorescence staining of explants is shown, confirming the successful removal of primary cilia from the explants, compared with untreated controls. Antiacetylated-α-actin (red) and anti-γ-tubulin (green) antibodies were used to identify the presence or absence of primary cilia. Scale bars are 7 μm in length. (b) quantitative real time–PCR analysis of Hh target genes PTCH1 and GLI1 showing an increased responsiveness to Shh ligand activation in chloral hydrate treated samples (red) compared with untreated controls (blue). Cyclopamine did not have a suppressive effect on the Hh pathway in explants lacking primary cilia. (c) Control explants showed increased proliferation (bromodeoxyuridine (BrDU)) and reduced apoptosis (Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)) following Hh pathway activation by Shh ligand. Chloral hydrate treated explants showed a comparatively enhanced effect on proliferation and apoptosis following Shh treatment, which could not be effectively inhibited by Cyclopamine. All error bars represent 95% confidence intervals, with the asterisk indicating a statistical significance of P<0.05.
Cilia-deficiency in the mouse results in an increased incidence of tumors and Hh pathway activation in growth plate chondrocytes
To investigate whether the loss of cilia would also alter the cartilage tumor phenotype in a mouse model of enchondroma, we crossed Col2A1-Gli2 mice with Ift88+/− mice. Col2A1-Gli2 mice develop benign cartilage tumors similar to that of human enchondromas.18, 19 Homozygous mutant Ift88orpk mice exhibit reduced expression of the intraflagellar transport protein Ift88, required for ciliary assembly. These mice frequently succumb to various developmental defects that lead to mid-gestational death.8 Therefore, we analyzed the adult growth plate phenotype of heterozygotes. Despite the fact that Ift88+/− mice still possess a wild-type (WT) allele, these mice display a significant reduction in proper ciliogenesis within developing growth plates compared with WT and Col2A1-Gli2 mice (Supplementary Figure 2). Quantification of ciliary incidence demonstrated an average of 86 and 76% of chondrocytes that possess a primary cilium in WT and Col2A1-Gli2 mice, respectively. In contrast, Ift88+/− mice was observed to have an average frequency of 31.6% of growth plate chondrocyte cilia, resulting in an ∼50% reduction in primary cilia incidence.
Surprisingly, cilia-deficient Ift88+/− mice developed enchondromas similar to that of Col2A1-Gli2 littermates (Figures 3a–c). As well, tumor incidence of both Ift88+/− and Col2A1-Gli2 occurred at the same frequency. However, double transgenic Col2A1-Gli2; Ift88+/− mice developed enchondromas at a higher frequency, which was determined to be significantly different from both Col2A1-Gli2 and Ift88+/− mice (P<0.05, Figures 3d and e). Compared with WT limbs, increased expression of Hh target genes was detected from Ift88+/− growth plate tissue that was comparable with that of Col2A1-Gli2 mice (Figure 3f). In comparison, double transgenic Col2A1-Gli2;Ift88+/− mice display an additive increase in Hh target gene expression.
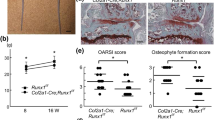
Primary cilia deficiency promotes tumorigenesis in the mouse. Histological analysis of adult femur was performed using safranin O and H&E staining to observe enchondromas that developed. (a) Safranin O stains cartilaginous tissue red that allows for clear identification of growth plate cartilage and enchondroma formation. The major features of the adult bones of mice are shown, demonstrating a typical cartilage lesion that develops below the growth plate and within the metaphyseal region. Higher magnification of H&E stained slides of enchondromas that formed in (b) Col2A1-Gli2 (c) Ift88+/− and (d) Col2A1-Gli2; Ift88+/− mice shows similarities in tumor size and cellularity to each other. (d) Tumor incidence of transgenic mouse models. (e) Comparison of Hh target gene expression (Gli1, Ptch1) of growth plate tissue from transgenic mice using qRT–PCR. A twofold increase in expression is observed in the double transgenic Col2A1-Gli2; Ift88+/− limbs compared with either Col2A1-Gli2 or Ift88+/− limbs. All error bars represent 95% confidence intervals, with the asterisk indicating a statistical significance of P<0.05.
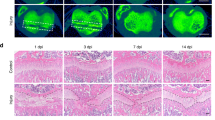
To determine whether murine enchondroma show a similar frequency of primary cilia to that observed in human tumors, adult limbs of transgenic mice were analyzed. Taking advantage of the fact that mice continue to maintain a growth plate throughout adulthood, growth plate chondrocytes were used as a normal control for comparison with enchondroma cells. In contrast to growth plate chondrocytes of adult bones that display the presence of cilia in the majority of cells (66.7±5.0%), enchondromas that developed in Col2A1-Gli2 mice possessed primary cilia in only a small fraction of tumor cells (24.3±6.4%, Figure 4). Both Ift88+/− and Col2A1-Gli2; Ift88+/− adult limbs demonstrated a low frequency of ciliated cells in enchondromas that were comparable to the ciliary incidence observed in their respective growth plate chondrocytes. Therefore, a deficiency in ciliogenesis can initiate tumor formation in our mouse model, and a low frequency of ciliated cells is a characteristic feature of enchondromas and chondrosarcomas.
Col2A1-Gli2 mouse enchondroma cells are deficient in primary cilia. Adult limb bones of mice possess a growth plate that does not undergo closure at maturity. (a) Growth plate chondrocytes of adult limbs present primary cilia in the majority of cells in WT and Col2A1-Gli2 mice, while less that 20% of primary cilia were detected from growth plate chondrocytes of Ift88+/− and Col2A1-Gli2; Ift88+/− limbs. The proliferative columnar region of the growth plates are represented here. (b) Conversely, tumor cells from enchondromas display a reduced frequency of primary cilia compared with their respective growth plate chondrocytes. Antiacetylated-α-tubulin (red) and anti-γ-tubulin (green) antibodies were used to identify primary cilia. Scale bars are 18 μm in length. Higher magnification images are provided from outlined boxed regions. (c) Quantification of primary cilia incidence is summarized, indicating a reduction in primary cilia incidence in enchondroma compared to normal growth plate chondrocytes. All error bars represent 95% confidence intervals, with the asterisk indicating a statistical significance of P<0.05.
A lack of cilia alters proliferation and apoptosis in the growth plate
As enchondromas that develop in mice are small, quantification of cellular changes has proven elusive. As such, the influence of Gli2 overexpression or ciliary-deficiency on chondrocyte behavior was investigated in the growth plate of developing limbs. Given the distinct phases of chondrocyte development present within the growth plate, this provides a reliable representation of the changes occurring in chondrocyte proliferation, differentiation and apoptosis.19 Limbs of mice harvested at postnatal day 1–3 were analyzed for cellular changes in growth plate chondrocytes. The percentage of cells stained positively for PCNA was used as an indicator of chondrocyte proliferation (Figure 5a). There was an increase in the proportion of PCNA-positive cells in the resting and proliferative zones of Col2A1-Gli2 and Ift88+/− limbs compared with WT littermates. There was also an increase in the length of the proliferative zone in these mice (Figure 5c). Furthermore, Col2A1-Gli2; Ift88+/− limbs showed an even greater increase in the proportion of proliferative cells and length of the proliferative zone. To assess changes in the proportion of chondrocytes differentiating to type X collagen expressing hypertrophic cells, the length of the hypertrophic zone was measured. Although a decrease in length was observed in hypertrophic zone of Col2A1-Gli2 and Ift88+/− limbs, no further reduction in length was shown in Col2A1-Gli2;Ift88+/− limbs. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was also performed to determine the rate of chondrocyte apoptosis within the growth plate. Col2A1-Gli2 and Ift88+/− limbs did not show a significant reduction in apoptotic cells compared with WT littermates, as indicated by the number of TUNEL-positive cells below the terminal hypertrophic zone (Figure 5b). However, in combination, Col2A1-Gli2; Ift88+/− limbs showed a significant reduction in apoptosis. The increase in proliferation and reduced apoptosis correlates with an upregulation of Hh target gene expression in our transgenic mice.
An increased frequency of tumor formation in (Col2A1-Gli2); Ift88+/− mice is associated with changes in chondrocyte proliferation and apoptosis during growth plate development, Developing limbs from early postnatal mice were compared for changes in proliferation (PCNA) and apoptosis (TUNEL). (a) Growth plate chondrocytes from the distal femur show an increase in proliferation in Col2A1-Gli2 and Ift88+/− limbs compared to WT littermates, which is further increased in Col2A1-Gli2; Ift88+/− limbs. The section shows the junction of the resting and proliferating zones of the growth plate ‘P’ labels a positively stained cells for PCNA, and ‘N’ labels a cell that has not stained (negative for staining). Graphically the resting and proliferating zones were analyzed separately, and the percentage of PCNA stained cells is shown as the mean and 95% confidence interval for each zone. (b) At the cartilage-bone interface, terminal hypertrophic chondrocytes undergo programmed cell death that is identified by TUNEL-positive cells. There was no change in the number of TUNEL-positive cells detected in Col2A1-Gli2 and Ift88+/− limbs compared to WT littermates, however, WT compared with Col2A1-Gli2; Ift88+/− limbs show a significant reduction in apoptosis. (c) Col2A1-Gli2; Ift88+/− mice show a significant increase in the length of their proliferative zone compared with WT mice. Hypertrophic zone lengths were reduced in Col2A1-Gli2 and Ift88+/− limbs compared with WT littermates, but no further differences were observed in double transgenic Col2A1-Gli2; Ift88+/−. All error bars represent 95% confidence intervals, with the asterisk indicating a statistical significance of P<0.05.
Primary cilia are required for GLI processing in chondrosarcomas and growth plate chondrocytes
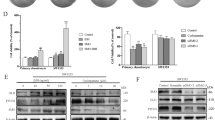
Previous studies show that the primary cilia are important in the processing of GLI proteins to activator or repressor forms.20, 21 GLI2 is primarily inactivated through proteosome-mediated degradation. In contrast, GLI3 functions can be processed into an activator and repressor form by protein truncation. Using western blot analysis of chondrosarcoma explants, chloral hydrate treated samples showed reduced expression of the GLI3 repressor form, compared with untreated controls (Figure 6a). Furthermore, growth plate chondrocytes extracted from IFT88+/− mice also demonstrated reduced Gli3 repressor protein compared with WT littermates (Figure 6b). Thus, primary cilia are important for normal GLI possessing in chondrocytes from the developing growth plate and chondrosarcoma.
Expression of Gli3 transcription factors in chondrocytes of primary chondrosarcoma and murine growth plates. (a) Primary culture chondrosarcoma explants were treated with chloral hydrate, followed either by Shh and/or cyclopamine. Actin was used as a loading control. Truncated Gli3-repressor protein expression is nearly absent in chloral hydrate treated cells compared with controls, while comparative levels of full-length Gli3 is observed across all samples. (b) A graphical representation showing means and 95% confidence intervals of the ration of GLI3R/GLI3FL levels. An asterisks over a bar indicates a significant difference from controls. (c) Growth plate chondrocytes from dissected limbs of neonatal mice exhibit reduced expression of the Gli3 repressor form in Ift88+/− mice compared with Wt littermates. These results were replicated from three independent experiments.
Aberrant activation of the Hh pathway induced by primary cilia deficiency is associated with degeneration of the articular cartilage
Hh signaling activation is known to cause articular cartilage degeneration.10 Chondrocyte-specific ablation of Ift88, also induces early markers of osteoarthritis and activation of the Hh pathway as a result of reduced Gli3 repressor to activator ratio.11 We analyzed the expression of osteoarthritis markers at the articular surface of our cilia-deficient mouse models in 4 month old mice (Supplementary Figure 3). Ift88+/− mice showed increased expression of Collagen X and decreased intensity of staining of safranin O of the articular cartilage. The severity of changes observed was similar to that of Col2A1-Gli2 limbs, while Col2A1-Gli2; Ift88+/− mice showed a more severe osteoarthritis phenotype. The articular cartilage of 4 month old mice was graded using the Osteoarthritis Research Society International (OARSI) methodology for comparison between transgenic genotypes.22 Col2A1-Gli2 and Ift88+/− limbs demonstrated characteristics associated with grade 1 features, including chondrocyte clusters, absence of chondrocytes within chondrons and increased chondrocyte hypertrophy. Conversely, Col2A1-Gli2; Ift88+/− limbs were largely associated with grade 2 criteria. In addition to the aforementioned characteristics of grade 1 histopathology, the articular cartilage of Col2A1-Gli2; Ift88+/− mice demonstrate persistent depletion of safranin O matrix staining, superficial zone discontinuity, and an apparent increase in chondrocyte hypertrophy as indicated by type X collagen staining. Thus, loss of primary cilia in chondrocytes results in worsening of the cartilage degeneration phenotype in combination with Gli2 overexpression, supporting the notion that there is a higher levels of Hh activity in the absence of cilia, and this has functional implications (an increased severity of osteoarthritis) in addition to the role in neoplasia.
Discussion
In this study we report that the majority of cells within chondrosarcomas and enchondromas lack primary cilia, suggesting an important role for cilia suppressing the neoplastic phenotype. Analysis of the adult limbs from Ift88+/− mice reveals that a ciliary deficiency alone is sufficient for the development of rests of benign chondrocytes to persist in the metaphyseal portion of bone. These enchondroma-like rests of chondrocytes are phenotypically similar to those that develop in the Hh-activated Gli2 mouse model, indicating that primary cilia has an important role in regulating terminal differentiation in the growth plate, as these chondrocyte rests most likely develop from growth plate chondrocytes that fail to undergo terminal differentiation. We found that Gli2-induced tumorigenesis is exacerbated in the absence of proper functional cilia. This increase in tumor formation is correlated with further elevation of Hh signaling activation. This finding is similar to that observed in some cancers, such as clear cell renal cell carcinoma, breast cancer, melanoma, basal cell carcinoma, MB and pancreatic cancer, which demonstrate a reduction in primary cilia in neoplastic cells,12, 14, 15, 16, 17, 23 and are associated with activation of the Hh pathway.
Opposing roles for primary cilia in tumorigenesis have been demonstrated in some of these Hh-induced cancers. Both basal cell carcinoma and MB can develop in mice as a result of activating mutations of the Hh pathway. Ciliary ablation strongly inhibits tumorigenesis of both tumor types, when induced by an activated form of Smo (SmoM2). In contrast, loss of primary cilia accelerates tumorigenesis in tumors induced by activated Gli2 (GLI2ΔN).12, 16 Therefore, the primary cilium potentiates ligand-dependent oncogenic mutations of the Hh pathway, but suppresses downstream oncogenic events. These seemingly conflicting results can be explained by the role of primary cilia in amassing the necessary components for efficient Gli processing and degradation.20, 24, 25, 26 The loss of the primary cilium results in the inefficient processing of Gli3 to its repressor form (Gli3-R), thus allowing for uncontrolled activation of the Hh pathway.12, 16
We compared the frequency of primary cilia in human tumors to human articular chondrocytes, as these are more readily available than growth plate chondrocytes, and come from individuals that are of similar age as develop chondrosarcomas. Furthermore, primary cilia are present in most human articular chondrocytes, even in osteoarthritis, similar to the frequency observed in growth plates,9 so serve as a valid positive control. We also analyzed the frequency of cilia in the mouse growth plates and tumors, and found a similar frequency, thus providing further evidence that primary cilia are present a lower frequency in cartilage tumors.
Our findings in chondrosarcoma support the notion that the cilia are required for tumor cell Gli processing. The Gli3 repressor form is dramatically reduced following ciliary ablation in vitro. As a result, cilia-depleted explants have an enhanced sensitivity to Hh ligand stimulation and an increase in its tumor-promoting effects. Thus, chondrocytes can respond to ligand-dependent stimulation of the Hh pathway in the absence of primary cilia. This is supported by the analysis of transgenic mice with targeted ablation of Ift88 specifically in chondrocytes that appear normal at birth, but eventually demonstrate dwarfism caused by the merging of primary and secondary ossification centers.6 As Hh ligand expression is crucial for normal embryonic growth plate development, primary cilia must not be required for Hh signaling activation in chondrocytes, but rather function to attenuate the activity, and thus explains why they are important for longitudinal growth of the postnatal growth plate. In chondrosarcoma, the primary cilia partially suppresses Hh activity with ligand activation, thus the lack of cilia in most neoplastic cells could in part explain the activation of Hh signaling in cartilage tumors.
Interestingly, chondrosarcoma cells were resistant to the inhibitory effects of cyclopamine following the removal of cilia. As a Smo inhibitor, cyclopamine relies on the accumulation of Smo at the primary cilium for efficient suppression of the Hh pathway. Thus, ablation of primary cilia in chondrosarcoma results in inefficient processing of Gli3 repressor forms and prevents the concentration of Smo, which is necessary for Hh pathway inhibition by cyclopamine. We used chloral hydrate in explant cultures, as it disrupts the junction of the cilium and the basal body, and as such is the only agent available, which inactivates cilia. However, chloral hydrate has off-target effects, such as on intracellular calcium level and mitosis rate.27 We used both human explant cultures, as well as genetically modified mice to asses the function of cilia in the regulation of Hh signaling in chondrocytes. While it is possible that the results of experiments using chloral hydrate could be due to off-target effects, the agreement between data from genetically modified mice and explant experiments, suggests that our results in cultures are most likely related to the inhibition of primary cilia function.
On the basis of genomic profiling analyses, copy number losses and loss of heterozygosity are frequently reported in chromosomal regions 9p13.1-pter and 13q11-q34 that include IFT74 and IFT88; genes, which are required for proper ciliogenesis.28, 29 Given that there is no known mutational basis for Hh-pathway activation in chondrosarcoma, this raises the possibility that defects in genes required for normal ciliogenesis has a role in Hh-induced tumorigenesis. This is suggested in renal carcinomas, which display a reduced frequency of primary cilia that is associated with inactivation of the von Hippel–Lindau tumor suppressor gene and constitutive activation of the Hh pathway.14, 30
It is possible that the activation of Gli mediated transcription in chondrocytes can suppress cilia function. Indeed, oncogenic events are shown to repress cilia formation in a pancreatic cell line, such that ciliogenesis was restored following the inhibition of Kras effector pathways.15 We observed an incidence of cilia in the developing growth plate of Col2A1-Gli2 mice is similar to that of WT mice. However, cells from Gli2-induced enchondroma-like lesions demonstrate a depletion of primary cilia compared with adjacent growth plates. Such cells might have an activation of Hh signaling and escape the controlled regulation of chondrocyte differentiation that eventually leads to cell death, and thus remaining behind as benign lesions following growth plate closure.
As a regulator of the Hh pathway, primary cilia are important for Gli processing and degradation, and thus effectively suppresses downstream Hh signaling events in neoplastic chondrocytes. The loss of primary cilia is sufficient to cause the activation of Hh signaling, and as a result some cells will evade terminal differentiation and remain as metaphyseal enchondroma-like lesions. It also causes degeneration of articular cartilage, similar to that seen in osteoarthritis. A lack of cyclopamine responsiveness in the absence of cilia raises the possibility that the cilia presence can be used to determine, which cartilage tumors may be responsive to Hh modulation. Our findings strongly support the notion that cilia-defective mechanisms are involved in the induction or maintenance of cartilage neoplasia.
Materials and methods
Human samples
Informed consent was obtained from all patients before surgery. These studies were approved by the Mount Sinai Hospital Research Ethics Review Board and The Ethics Review Board Research Institute Hospital for Sick Children. Ten chondrosarcomas, five enchondromas and five normal articular cartilage samples were analyzed for the presence of primary cilia. Chondrosarcoma samples were also prepared for explant organ culture immediately after surgical excision as previously reported.31 Each explant experiment was analyzed in triplicate.
Mice
A mouse model of enchondromatosis, in which the Hh-activated growth factor, Gli2, is expressed in growth plate chondrocytes driven by the regulatory elements of type 2 collagen, Col2A1-Gli2, was to study benign cartilage neoplasia.18 These mice develop tumors driven by a molecular mechanism that is a common feature in enchondromas, and as such are an applicable model for the human disorder. Ift88orpk mice possess a hypomorphic allele of the Ift88 gene that expresses the Ift88 protein, required for ciliogenesis.8 The embryonic and early postnatal growth plate phenotype is analogous to that observed in transgenic mice with chondrocyte-specific ablation of Ift88.6, 32 Cilia-deficient Ift88+/− mice were crossed with Col2A1-Gli2 mice using a previously reported crossing strategy.33 The tumor phenotype between at least eight littermates of each genotype was compared at 4 months of age. Analysis of differences in the growth plate phenotype was also analyzed in 1 day old pups.
Explant culture
Human chondrosarcoma samples were prepared as 2 mm cubes and cultured in Dulbecco’s modified Eagle's medium (DMEM) containing 10% bovine serum albumin (BSA), and antibiotic–antimycotic solution (Wisent, St-Bruno, QC, Canada ). Explants were treated with 10 mM chloral hydrate (Sigma-Aldrich, Oakville, ON, Canada) overnight to remove cilia using a previously reported protocol.34 Samples were subsequently cultured in fresh media for 24 h, followed by treatment with 5 μg/ml Shh ligand (R&D Systems, Minneapolis, MN, USA) and/or 10−4 M cyclopamine (Biomol Research Laboratories, Inc. Plymouth Meeting, PA, USA) overnight, as reported elsewhere.1 For proliferation experiments, explants were incubated with bromodeoxyuridine (BrDU) for the final 12 h of culture. Three replicates were performed for each treatment per sample.
Quantitative real–time PCR
RNA was isolated from at least three independent experiments and was analyzed in separate PCRs. Gene expression between samples was calculated using the  method.35 All human and mouse Taqman primers were obtained from Applied Biosystems (Life Technologies, Carlsbad, CA, USA). Either the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or β-actin gene was used for target gene normalization.
method.35 All human and mouse Taqman primers were obtained from Applied Biosystems (Life Technologies, Carlsbad, CA, USA). Either the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or β-actin gene was used for target gene normalization.
Western blot
Protein was isolated from human chondrosarcoma explants and mouse neonatal growth plate chondrocytes using TRIZOL Reagent (Life Technologies), according to the manufacturer’s instructions. Expression level of both full-length and truncated forms of Gli3 was determined using a Gli3 antibody (H-280, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:50 dilution factor. The membranes for the western blot were probed using an antiactin antibody (1:10 000; Calbiochem, Billerica, MA, USA) as a loading control.
Histological analysis, immunohistochemistry and immunofluorescence
Samples were fixed in 4% paraformaldehyde, treated with 20% EDTA for decalcification (for adult bone only), embedded in paraffin and sectioned for histological evaluation. Safranin O and hematoxylin and eosin staining was performed using standard techniques. ColX staining was used as a marker for chondrocyte terminal differentiation, by incubating the sections with a 1:50 dilution of antihuman recombinant ColX (Quartett Immunodiagnostika Biotechnologie, GMBH, Berlin, Germany) at 4 °C overnight. TUNEL staining was used to detect apoptotic cells in tissue sections. Deparaffinized sections were stained using the Apoptag Plus Peroxidase In Situ Apoptosis Detection Kit (Millipore, Billerica, MA, USA), according to the manufacturer’s instructions. PCNA, Ki67 or BrDU was used as a marker for proliferation at a dilution of 1:200 (Cell Signaling, Boston, MA, USA) or using the BrDU staining kit (Invitrogen, Carlsbad, CA, USA), respectively.
Quantification of primary cilia
The presence of cilia was confirmed using a mouse antiacetylated α-tubulin antibody (T7451, Sigma-Aldrich Inc.) at a dilution of 1:1500. Double staining with a rabbit anti-γ-tubulin antibody (AK-15, Sigma-Aldrich Inc.) to identify microtubule organizing centers or centrioles was performed at a 1:1000 dilution. Secondary antibodies were used at a 1:100 dilution (donkey antimouse Alexa 594 and donkey antirabbit Alexa 488; Invitrogen). Fluorescent staining was analyzed using a Quorum Spinning Disk Confocal Microscope. Given the three-dimensional morphology of primary cilia, the Volocity Imaging software (Perkin Elmer Inc, Waltham, MA, USA.) was used to compile Z-stacked images for accurate identification of ciliary projections from the cell. Paraffin embedded samples were cut in 5 μm sections and scanned in their full depth to evaluate the presence of cilia. Ten fields of randomly selected views from each zone of the growth plate or tumor sample were analyzed from each specimen. Only cells with visible cytoplasm and nuclear staining with 4',6-diamidino-2-phenylindole (DAPI) were determined to be viable. Colocalization of with α-tubulin and γ-tubulin was used to correctly identify primary cilia. Positive control tissue samples with established localization and incidence of primary cilia (that is,. lung and kidney) were used in each experiment.
Grading of osteoarthritis
The articular cartilage at the knee joint was assessed between at least eight littermates of each genotype at 4 month old mice. Decalcified adult limbs were cut in 5 μm thickness sections and graded at 50 μm intervals across the entire joint. Both safranin O and H&E stained slides were analyzed under blinded conditions by two observers using the OARSI method of scoring.22 The OARSI method is demonstrated to be reliable and reproducible for scoring osteoarthritis.36
Statistical analysis
Means and 95% confidence intervals were calculated. The Student’s t-test or one-way analysis of variance (ANOVA) was used for comparison of data sets. A P-value of <0.05 was used as a threshold for statistical significance. Correlation between proliferation and the incidence of primary cilia was assessed by Spearman’s rank correlation, a non-parametric method of linear regression that does not assume the normal distribution of variables.
References
Tiet TD, Hopyan S, Nadesan P, Gokgoz N, Poon R, Lin AC et al. Constitutive hedgehog signaling in chondrosarcoma up-regulates tumor cell proliferation. Am J Pathol 2006; 168: 321–330.
Yan T, Angelini M, Alman BA, Andrulis IL, Wunder JS . Patched-one or smoothened gene mutations are infrequent in chondrosarcoma. Clin Orthop Relat Res 2008; 466: 2184–2189.
Goetz SC, Anderson KV . The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 2010; 11: 331–344.
Tobin JL, Beales PL . The nonmotile ciliopathies. Genet Med 2009; 11: 386–402.
de Andrea CE, Wiweger M, Prins F, Bovee JV, Romeo S, Hogendoorn PC . Primary cilia organization reflects polarity in the growth plate and implies loss of polarity and mosaicism in osteochondroma. Lab Invest 2010; 90: 1091–1101.
Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R . Development of the postnatal growth plate requires intraflagellar transport proteins. Dev Biol 2007; 305: 202–216.
Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R et al. Intraflagellar transport is essential for endochondral bone formation. Development 2007; 134: 307–316.
McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA . Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol 2007; 26: 234–246.
McGlashan SR, Cluett EC, Jensen CG, Poole CA . Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev Dyn 2008; 237: 2013–2020.
Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med 2009; 15: 1421–1425.
Chang CF, Ramaswamy G, Serra R . Depletion of primary cilia in articular chondrocytes results in reduced Gli3 repressor to activator ratio, increased Hedgehog signaling, and symptoms of early osteoarthritis. Osteoarthritis Cartilage 2011; 20: 152–161.
Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A . Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med 2009; 15: 1062–1065.
Moser JJ, Fritzler MJ, Rattner JB . Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer 2009; 9: 448.
Schraml P, Frew IJ, Thoma CR, Boysen G, Struckmann K, Krek W et al. Sporadic clear cell renal cell carcinoma but not the papillary type is characterized by severely reduced frequency of primary cilia. Mod Pathol 2009; 22: 31–36.
Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M . Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res 2009; 69: 422–430.
Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 2009; 15: 1055–1061.
Yuan K, Frolova N, Xie Y, Wang D, Cook L, Kwon YJ et al. Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J Histochem Cytochem 2010; 58: 857–870.
Hopyan S, Gokgoz N, Poon R, Gensure RC, Yu C, Cole WG et al. A mutant PTH/PTHrP type I receptor in enchondromatosis. Nat Genet 2002; 30: 306–310.
Ho L, Stojanovski A, Whetstone H, Wei QX, Mau E, Wunder JS et al. Gli2 and p53 cooperate to regulate IGFBP-3- mediated chondrocyte apoptosis in the progression from benign to malignant cartilage tumors. Cancer Cell 2009; 16: 126–136.
Liu A, Wang B, Niswander LA . Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 2005; 132: 3103–3111.
Hsu SH, Zhang X, Yu C, Li ZJ, Wunder JS, Hui CC et al. Kif7 promotes hedgehog signaling in growth plate chondrocytes by restricting the inhibitory function of Sufu. Development 2011; 138: 3791–3801.
Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage 2006; 14: 13–29.
Kim J, Dabiri S, Seeley ES . Primary cilium depletion typifies cutaneous melanoma in situ and malignant melanoma. PLoS One 2011; 6: e27410.
Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK . Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 2005; 1: e53.
Huangfu D, Anderson KV . Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA 2005; 102: 11325–11330.
May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol 2005; 287: 378–389.
Lee GM, Diguiseppi J, Gawdi GM, Herman B . Chloral hydrate disrupts mitosis by increasing intracellular free calcium. J Cell Sci 1987; 88 (Part 5): 603–612.
Hallor KH, Staaf J, Bovee JV, Hogendoorn PC, Cleton-Jansen AM, Knuutila S et al. Genomic profiling of chondrosarcoma: chromosomal patterns in central and peripheral tumors. Clin Cancer Res 2009; 15: 2685–2694.
Pansuriya TC, Oosting J, Krenacs T, Taminiau AH, Verdegaal SH, Sangiorgi L et al. Genome-wide analysis of Ollier disease: is it all in the genes? Orphanet J Rare Dis 2011; 6: 2.
Dormoy V, Danilin S, Lindner V, Thomas L, Rothhut S, Coquard C et al. The sonic hedgehog signaling pathway is reactivated in human renal cell carcinoma and plays orchestral role in tumor growth. Mol Cancer 2009; 8: 123.
Chen JK, Taipale J, Young KE, Maiti T, Beachy PA . Small molecule modulation of Smoothened activity. Proc Natl Acad Sci USA. 2002; 99: 14071–14076.
Serra R . Role of intraflagellar transport and primary cilia in skeletal development. Anat Rec (Hoboken) 2008; 291: 1049–1061.
Poon R, Smits R, Li C, Jagmohan-Changur S, Kong M, Cheon S et al. Cyclooxygenase-two (COX-2) modulates proliferation in aggressive fibromatosis (desmoid tumor). Oncogene 2001; 20: 451–460.
Praetorius HA, Spring KR . Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 2003; 191: 69–76.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Pauli C, Whiteside R, Heras FL, Nesic D, Koziol J, Grogan SP et al. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthritis Cartilage 2012; 20: 476–485.
Acknowledgements
Wewould like to acknowledge the Canadian Institutes of Health Research for supporting this research project through operating grant no, MOP- 37913. Funding by the University of Toronto Fellowship and Hospital for Sick Children (RESTRACOMP) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Ho, L., Ali, S., Al-Jazrawe, M. et al. Primary cilia attenuate hedgehog signalling in neoplastic chondrocytes. Oncogene 32, 5388–5396 (2013). https://doi.org/10.1038/onc.2012.588
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.588
Keywords
This article is cited by
-
Freeing the brake: Proliferation needs primary cilium to disassemble
Journal of Biosciences (2020)
-
Ciliary signalling in cancer
Nature Reviews Cancer (2018)
-
A spiny Hedgehog
Nature Reviews Cancer (2013)
-
Homeostatic Mechanisms in Articular Cartilage and Role of Inflammation in Osteoarthritis
Current Rheumatology Reports (2013)