Abstract
Antibiotic resistance is increasingly affecting the management of infectious diseases. The prescribing clinician is not only important to the development of the problem but also central to its solution. This article addresses the current weaknesses in the information systems and the evidence base that support prescribing. Remedies necessary for improvements in prudent prescribing include better guidance in managing specific diseases where resistance is of prognostic significance and also increasing diagnostic precision.
Similar content being viewed by others
Main
The increasing prevalence of antibiotic-resistant microorganisms is now an issue of major public concern. Newspaper headlines and media reports regularly feature details of their impact on individuals as well as raising the spectre of widespread and untreatable drug-resistant infections. The breadth and significance of antibiotic-resistant microorganisms is becoming increasingly apparent. Examples include outbreaks of multiply antibiotic-resistant pathogens in intensive care or neonatal units; the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in hospitals and, more recently, nursing homes; the global occurrence of drug-resistant malaria in tropical and sub-tropical regions; and drug-resistant tuberculosis, with its links to poverty, HIV and social exclusion.
Reports on antibiotic resistance and concerns raised by infection specialists over the past fifty years were largely unheeded until the mid-1990s when the significance of the problem achieved political recognition. In 1995, the American Society for Microbiology1, and in 1998, a House of Lords Select Committee in the United Kingdom2, issued reports that have been influential in establishing national strategies. In the United States, a task force was established3. The European Union also published its strategy4 and addressed the problem through a series of conferences on antibiotic resistance, such as those held in Copenhagen5 and Rome6. The latter was organized by The European Society of Clinical Microbiology and Infectious Diseases on behalf of the Italian Government, and addressed the issue of applying research-led solutions to the control of antibiotic resistance.
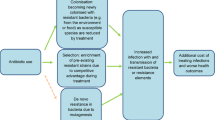
The current strategy for the control of antibiotic resistance, which has been adopted by many countries and was encapsulated in a World Health Organization (WHO) report7, is based on six main themes (Box 1). Improved surveillance of microorganisms aims to provide a more accurate definition of resistance rates as well as providing data on the prescribing of antibiotics. Both are key to developing more effective prescribing strategies and monitoring their impact. At present, too little is known about the prevalence of drug-resistant microorganisms in the community, where 80% of antibiotic prescribing takes place. In hospitals, there is patchy information on prescribing patterns despite better information on the rates of resistance8. Other components of the strategy to control antibiotic resistance correctly emphasize the need to encourage new drug and vaccine development, as well as highlighting the importance of promoting the principles and practice of infection control and effective hygiene within hospitals and among the public at large. Education is central to changing public and professional perceptions9 and reinforcing the key message in controlling antibiotic resistance, namely the prudent use of these agents by prescribing practitioners.
Prudent prescribing embodies the science and practice of medicine. A definition from the UK Department of Health reads as follows: “The use of antimicrobials in the most appropriate way for the treatment or prevention of human infectious diseases, having regard to the diagnosis (or presumed diagnosis), evidence of clinical effectiveness, likely benefits, safety, cost (in comparison with alternative choices) and propensity for the emergence of resistance.” Most clinicians would endorse the importance of prudent prescribing, but in reality there is ample evidence to indicate that there is excessive and indeed inappropriate use, such as in surgical prophylaxis10. Although there is no simple relationship between use and the selection, emergence or dissemination of antibiotic-resistant microorganisms, there is considerable evidence to support a relationship between excessive use and antibiotic resistance11. In Europe, resistance rates among penicillin-resistant pneumococci are clearly related to high prescribing rates12,13. It is therefore important to analyse some of the issues and obstacles to prudent prescribing from the perspective of the prescriber. These will be discussed by addressing a series of pivotal questions.
What is antibiotic resistance?
This question is fundamental to a full understanding and discussion of the subject but is rarely defined within the context in which it is being addressed. Resistance can imply the identification of a genetic or biochemical mechanism that may or may not be expressed in a microorganism. When expressed, it can be recognized in a clinical microbiology laboratory by a reduced zone of inhibition or increased minimum inhibitory concentration (MIC) on susceptibility testing. Another view of resistance recognizes the importance of the pharmacokinetic (PK) and pharmacodynamic (PD) parameters of an antibiotic (discussed later)14. Failure to achieve a particular target concentration of an antibiotic at the site of infection can result in treatment failure and can therefore be recognized clinically. However, much prescribing is for mild to moderately severe infections that are frequently self-limiting, and in this situation it is difficult to recognize antibiotic-resistant infections clinically. The final important element of resistance is its spread within both nosocomial and community settings.
What is the impact of resistance?
The impact of antibiotic resistance on prescribing practice over the past 40 years for a cross-section of diseases and microorganisms is summarized in Table 1. In previous years, considerable reliance was placed on the aminopenicillins (ampicillin and amoxicillin) and injectable cephalosporins. However, resistance has resulted in increasing reliance on the fluoroquinolones to which resistance is now emerging rapidly worldwide among common pathogens, notably Streptococcus pneumoniae and Escherichia coli 15.
How common is antibiotic resistance?
There have been many national and international surveys of the rates at which antibiotic resistance develops and the geographical variation of antibiotic-resistant pathogens. The European Antibiotic Resistance Surveillance System (EARSS, see the Online links box) provides regular reports on trends in resistance that are based on data from national surveillance systems. Likewise, the compulsory notification of MRSA bloodstream infections in England has provided more accurate information on recent trends in hospital isolates16. However, although prescribers are generally aware of the problem of increasing antibiotic resistance, they are rarely able to reliably predict when this might frustrate disease management in an individual patient. For example, penicillin resistance among S. pneumoniae is a global problem with varying geographical impact, and this pathogen is also associated with various manifestations of disease and disease severity. In the United Kingdom, the burdens of disease and mortality vary widely between pneumococcal septicaemia and pneumonia and yet pathogens that are highly resistant to penicillin are isolated from very few patients. Recommendations exist for alternative antibiotic management17, but knowing when to adopt these recommendations at local or national level is challenging, even in countries with high rates of drug-resistance, such as Spain.
Microbiological surveillance remains an inexact process. There are many limitations in the current data, which continue to frustrate its practical application in guiding prescribing practice among clinicians whose primary responsibility lies in the management of disease18. Antibiotic resistance is the unseen challenge that can frustrate this goal. It is important to understand why the current surveillance systems have such limited benefit. The limitations not only relate to the population and nature of the infections sampled, but also to how such information might best be used (Box 2).
There is a pressing need to define the true rates of resistance by disease in order to better support the appropriate choice of therapy. Currently, in Europe and North America there are high rates of resistance among S. pneumoniae to penicillin, erythromycin and other antibiotics, and yet β-lactams and macrolides continue to be widely prescribed for community respiratory infections and their use is supported by authoritative guidelines19,20. There is often a clear dichotomy between antibiotic-resistant pathogens that have been defined in vitro and their in vivo expression in the form of definable diseases and syndromes that are treated by clinicians. There is therefore an urgent need to relate resistance data with disease management to develop guidelines that support prescribing practice18. Few would argue against a change in initial therapeutic choice for life-threatening diseases such as pneumococcal meningitis21; however, the frequency of resistance that should guide the choice of therapy for severe, yet non-life-threatening, hospital-managed infections or mild, community-managed infections, such as those affecting the urinary tract, is not defined. Addressing this absence of sound guidance should be a priority for healthcare research.
At present, microbiological surveillance data are mainly based on samples collected from hospitalized patients, particularly those considered most at risk of sepsis, such as those in high-dependency units (bone marrow, transplant, burns, renal, neonatal and intensive care units) and the elderly. This inevitably introduces selection bias to the data, since such patients are at increased risk of acquiring drug-resistant microorganisms or are likely to have received antibiotics either at the time of sampling or in the recent past, which increases their risk of carrying an antibiotic-resistant microorganism8. Unless an effort is made to sample systematically from 'spotter units' in the community and hospitals, estimates of the true prevalence and trends in resistance will remain unreliable. Furthermore, there is rarely any systematic attempt to correlate microbiological susceptibility data with disease or disease outcome, except in relation to uncommon infections such as meningitis. Translating this incomplete information into prescribing recommendations and policies inevitably introduces a degree of uncertainty and reduces its clinical utility.
Organisms of greatest concern?
For the prescriber, there is no simple answer to this question. Antibiotic-resistant pathogens are common and are increasing in frequency in both the community and in hospitals. Numerically, staphylococci and E. coli are the most frequently encountered bloodstream isolates from hospital-acquired infections, whereas S. pneumoniae and, again, E. coli, are seen in patients with infections that originate in the community. Multiple-antibiotic resistance is common among all three pathogens and yet the diseases with which they are associated present very different management requirements. Infections caused by penicillin-resistant pneumococci, apart from meningitis and otitis media, are still largely responsive to β-lactam agents; E. coli is primarily associated with intra-abdominal and urinary-tract infections and neonatal meningitis, and usually responds to third-generation cephalosporins or fluoroquinolones (contraindicated in children); MRSA on the other hand, causes a variety of clinical problems, ranging from post-operative wound and medical-device-associated infections to pneumonia, all of which have a high risk of bloodstream invasion. This is compounded by the predilection of MRSA for patients with compromised host defences, which further increases its medical importance because the choice of therapy is mainly limited to two classes of antibiotic — glycopeptides and oxazolidinones. In addition, the recent emergence of virulent strains of MRSA in the community, which are causing severe soft-tissue and respiratory infections in children and adults, is also of concern. Timely laboratory identification is therefore essential for appropriate management and containment.
Investigations based on epidemiological and surveillance data have identified several patient populations among community and hospitalized patients that are at risk of being colonized by resistant pathogens (Box 3). Additional risk factors can be identified, such as age, immunosuppressive therapy, surgical procedures and recent exposure to antibiotics. However, such information, although useful in population studies, is often unhelpful in guiding the clinician in the selection of initial empirical therapy in individual patients. It would be inappropriate to manage all patients that are considered to be at higher risk of antibiotic-resistant pathogens with an antibiotic regimen that targets every possible resistant pathogen, largely on grounds of cost and the potential for toxicity.
How are such infections recognized?
At present, the prescriber is dependent on collecting appropriate microbiological samples, which are subject to a series of laboratory investigations that are directed at isolating and confirming the nature and antibiotic susceptibility of a pathogenic organism. Current practice means that 24–48 hours elapse from sample collection to the issuing of a report. This inevitably reduces the potential usefulness of such investigations to guide initial therapeutic choice among hospital-managed patients. This situation is compounded in community-managed patients from whom microbiological samples are rarely routinely collected. The inevitable lack of diagnostic precision remains one of the greatest hurdles to appropriate prescribing and, in particular, to the early recognition of drug-resistant infections.
In an era of increasing drug resistance it is also important to note the occasional failure of diagnostic laboratories to accurately identify drug-resistant pathogens such as S. pneumoniae and E. coli owing to unsatisfactory sample processing, or the failure to introduce appropriate identification techniques as new resistance problems arise22,23,24.
What is the clinical importance?
It is not difficult to identify the likely clinical impact and outcome that would result from inappropriately managed infections caused by antibiotic-resistant organisms in an individual patient. Several studies over the past twenty years attest to the increased risk of death in inappropriately versus appropriately treated patients25,26. More specifically, the relative risks of a fatal outcome associated with hospital-acquired bloodstream MRSA infections compared with methicillin-sensitive S. aureus (MSSA) infections endorse these observations27,28 and make early recognition a major healthcare priority if patient outcomes are to be improved.
How can prescribing be improved?
Contrary to some sensational headlines and occasional professional bias, prescribing practice is not an area of universal medical mayhem. There is no shortage of formularies, prescribing policies and guidelines directed at the prescriber. Indeed, the multiplicity of recommendations adds to the information overload that many prescribing professionals have to cope with and which should ideally be readily available online (for example, at the National Electronic Library of Infection (NELI) web site; see the Online links box). Prescribing recommendations can be produced locally or nationally and incorporate, to varying degrees, surveillance data and published evidence on disease management. However, many recommendations are influenced by the acquisition costs of drugs, as well as local prescribing practice, with any attempt to control antibiotic resistance being a subsidiary consideration. Differences in prescribing recommendations even extend to evidence-based guidance for the management of the same disease29, which is indicative of the relative lack of robustness of the information base that is available to guide prescribing.
When considering how prescribing recommendations might either control or assist in preventing antibiotic-resistant infections, there is little published evidence to guide the prescriber. There are some examples of the 'ebb and flow' of drug-resistant pathogens in response to changes in prescribing practice11, but these observations are not commonly incorporated into prescribing practice nor rigorously studied in relation to disease outcome30.
As discussed above, the application of pharmacodynamic principles to predict in vivo efficacy has identified the risk of drug-resistant organisms emerging in situations where the target PK/PD parameters cannot be achieved in vivo14. There is an urgent need for appropriately designed studies of the full repertoire of licensed and commonly prescribed antibiotics (particularly generic agents, which account for most prescriptions worldwide) to establish the most appropriate dosage regimen for the management of common target diseases based on PK/PD determinants of efficacy. Such studies will probably need to be funded as a partnership between healthcare providers, grant-giving agencies and the pharmaceutical industry.
Faced with such a complex and multifaceted problem, the control of antibiotic resistance will require considerable ingenuity and investment if it is to be successful (Box 4). However, the prescriber and the professional advice given to, and the treatment of, the patient are central to this strategy. Education therefore has an important role in raising awareness of the increasing problem of drug resistance among the public and healthcare professionals, and of the manner in which these antibiotics should best be used to control human suffering, while maintaining their effectiveness for future generations.
Several public-health campaigns have been conducted in Europe and North America, with varying degrees of success, to raise public awareness about antibiotics and antibiotic resistance8 (for example, the 'Do Bugs Need Drugs?' project; see the Online links box). In the United Kingdom, the focus of a public and professional campaign has been to reduce the prescribing of antibiotics in the management of upper-respiratory-tract infections, which are largely viral in nature31. There has been a substantial reduction in the number of prescriptions, but this trend seems to have preceded this campaign and might have other explanations, such as the steady decline in recorded respiratory infections over the past decade32. The long-term success of any educational approach to antibiotic prescribing and resistance must continue to focus on the professional consultation between the prescriber and the patient and the need to repeatedly reinforce the key messages for the prudent prescribing of antibiotics (Box 5).
Agenda for change
As the prescriber is central to supporting the strategy for controlling antibiotic resistance, I return to an issue of fundamental importance, namely that of diagnostic precision. Currently, most (and in primary care nearly all) initial antibiotic prescribing is based on clinical assessment and empirical choice of therapy. This inevitably means that the true microbiological nature of the target infection is generally unknown at the point of prescribing and, in turn, any audit or assessment of response, which might improve future management for sensitive or resistant pathogens, is compromised. More specifically, and at its simplest level, the failure to distinguish bacterial from viral infections means that the latter will continue to be treated unnecessarily, thereby exposing the patient to the potential side-effects of an unnecessary prescription, as well as adding to the cost of medicines either to the individual or the healthcare provider. It is difficult to justify such a continued state of affairs, even apart from the current concerns about the risk of encouraging antibiotic resistance.
The global expenditure on medical diagnostics accounts for about 1–2% of the amount that is spent on antibiotic drugs. Incentives to develop clinically useful diagnostics, especially those that might influence initial patient assessment and the decision whether or not to prescribe an antibiotic, could have many benefits over and above those of simply reducing or containing the risks of antibiotic-resistant microorganisms (Box 6). The nature of such new diagnostics should be complementary to medical decision-making and need not be entirely directed at defining the exact nature of the microbiological cause of a particular disease, which has been the focus of much 'chip' technology so far. Diagnostics might be designed to have syndromic application in areas where prescribing practice remains particularly challenging — for example, throat infections, urinary-tract infections and cough and sputum with and without focal chest signs. Healthcare systems will need to define their requirements more precisely, while industry needs to be more innovative. Policy makers and governments should provide incentives and facilitate such developments.
In conclusion, prescribers are faced with a dilemma in which they are often viewed as the major cause of antibiotic resistance. However, as argued above, the nature of healthcare delivery, the widespread lack of robust prescribing support that encourages greater diagnostic precision, and in turn, the current limited value of much surveillance data to support prescribing, are important obstacles to prudent prescribing. Educational strategies certainly have an important role in supporting the general principles of good prescribing practice but cannot resolve the questions surrounding antibiotic resistance in the case of individual patient management. These are pressing questions that demand solutions if such an important medical technology as antibiotic therapy is to remain effective in the treatment and prevention of infectious disease for current and future generations.
References
American Society for Microbiology. Report of the ASM task force on antibiotic resistance. [online], <http://www.asm.org/ASM/files/CCPAGECONTENT/DOCFILENAME/ 0000005962/antibiot[1].pdf> (ASM, Washington DC, 1995).
House of Lords Select Committee on Science and Technology. Resistance to antibiotics and other antimicrobial agents. [online], <http://www.parliament.the-stationery-office.co.uk/pa/ld199798/ldselect/ldsctech/081vii/st0701.htm> (The Stationery Office, London, UK, 1998).
Interagency Task Force on Antimicrobial Resistance. Public health action plan to combat antimicrobial resistance. [online], <http://www.cdc.gov/drugresistance/actionplan/aractionplan.pdf> (CDC, Atlanta, 2001).
EU Ministers of Health. 2188th Council Meeting. Antibiotic resistance — a strategy against the microbial threat — Council Resolution. [online], <http://ue.eu.int/ueDocs/cms_Data/docs/pressData/en/Isa/ACF152.htm> (1999).
Ministry of Health, Ministry of Food, Agriculture and Fisheries. The Copenhagen Recommendations. Report from the invitational EU conference on the microbial threat. [online], <http://www.archive.official-documents.co.uk/document/cm41/4172/4172.pdf> (9–10 September, 1998).
Cornaglia, G. et al. Report from the European conference on the role of research in combating antibiotic resistance, 2003. Clin. Microbiol. Infect. 10, 473–497 (2004).
World Health Organization. WHO global strategy for the containment of antimicrobial resistance. [online], <http://www.who.int/emc-documents/antimicrobial_resistance/docs/EGlobal_Strat.pdf> (World Health Organization, Geneva 2001).
Finch, R. G. et al. Antibiotic resistance. J. Antimicrob. Chemother. 42, 125–128 (1998).
Finch, R. G. et al. Educational interventions to improve antibiotic use in the community: report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002. Lancet Infect. Dis. 4, 44–52 (2004).
Scottish Intercollegiate Guidelines Network (SIGN). Antibiotic prophylaxis in surgery. A national clinical guideline. Publication number 45 [online], <http://www.guideline.gov/summary/summary.aspx?doc_id=2911> (2000).
Livermore, D. et al. Can better prescribing turn the tide of resistance? Nature Rev. Microbiol. 2, 73–78 (2004).
Cars, O. et al. Variation in antibiotic use in the European Union. Lancet 357, 1851–1853 (2001).
Baquero, F. et al. Antibiotic consumption and resistance selection in Streptococcus pneumoniae. J. Antimicrob. Chemother. 50, 27–38 (2002).
Drusano, G. L. et al. Antimicrobial pharmacodynamics: critical interactions of 'bug and drug'. Nature Rev. Microbiol. 2, 289–300 (2004).
European Antimicrobial Resistance Surveillance System. Annual Report 2003. [online], <http://www.earss.rivm.nl/> (EARSS, 2003).
Annual report of the regional and national analyses of the MRSA surveillance scheme in England. The third year of regional and national analyses of the Department of Health's mandatory MRSA surveillance scheme in England: April 2001–March 2004. CDR Weekly 14, 1–6 [online], <http://www.hpa.org.uk/cdr/PDFfiles/2004/cdr2904.pdf> (2004).
Ziglam, H. M. et al. Penicillin-resistant pneumococci — implications for management of community-acquired pneumonia and meningitis. Int. J. Infect. Dis. 6 (Suppl.), S14–S20 (2002).
Finch, R. G. et al. Antibiotic resistance — from pathogen to disease surveillance. Clin. Microbiol. Infect. 8, 317–320 (2002).
Macfarlane, J. T. et al. The British Thoracic Society guidelines for the management of community-acquired pneumonia in adults. Thorax 56 (Suppl.), S1–S64 (2001).
Mandell, L. A. et al. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin. Infect. Dis. 37, 1405–1433 (2003).
Quagliarello, V. J. et al. Treatment of bacterial meningitis. N. Engl. J. Med. 336, 708–716 (1997).
Henwood, C. J. et al. Accuracy of routine susceptibility testing of Streptococcus pneumoniae in England and Wales. J. Antimicrob. Chemother. 47, 897–900 (2001).
Warren, R. E. et al. Simultaneous, bi-clonal outbreak of urinary tract infection by E. coli 025 strains with CTX-M-15: community and hospital effects in two English health districts. Abstracts of the 14th European Congress of Clinical Microbiology and Infectious Diseases, Prague, Czech Republic, Abstract P756, [online], <http://www.blackwellpublishing.com/eccmid14/abstract.asp?id=14399> (2004).
Brenwald, N. P. et al. An outbreak of a CTX-M-type β-lactamase-producing Klebsiella pneumoniae: the importance of using cefpodoxime to detect extended-spectrum β-lactamases. J. Antimicrob. Chemother. 51, 195–196 (2003).
Kollef, M. H. et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115, 462–474 (1999).
Ibrahim, E. H. et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118, 146–155 (2000).
Cosgrove, A. E. et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36, 53–59 (2003).
Melzer, M. et al. Is methicillin-resistant Staphylococcus aureus more virulent than methicillin-susceptible S. aureus? A comparative cohort study of British patients with nosocomial infection and bacteremia. Clin. Infect. Dis. 37, 1453–1460 (2003).
Finch, R. G. et al. Practical considerations and guidelines for the management of community-acquired pneumonia. Drugs 55, 31–45 (1998).
Metlay, J. P. et al. Outcomes in lower respiratory tract infections and the impact of antimicrobial drug resistance. Clin. Microbiol. Infect. 8 (Suppl.), S1–S11 (2002).
UK Standing Medical Advisory Committee (SMAC). The path of least resistance. [online], <http://www.advisorybodies.doh.gov.uk/smac1.htm> (Department of Health, UK, 1998).
Flemming, D. M. et al. The reducing incidence of respiratory tract infection and its relation to antibiotic prescribing. Br. J. Gen. Pract. 53, 778–783 (2003).
Acknowledgements
This article is based on an invited lecture to the 14th European Congress of Clinical Microbiology and Infectious Diseases, Prague, April 2004.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez
FURTHER INFORMATION
The European Society of Clinical Microbiology and Infectious Diseases
Alliance for the Prudent Use of Antibiotics
FDA antibiotic resistance fact sheet
Rights and permissions
About this article
Cite this article
Finch, R. Antibiotic resistance: a view from the prescriber. Nat Rev Microbiol 2, 989–994 (2004). https://doi.org/10.1038/nrmicro1049
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1049