Key Points
-
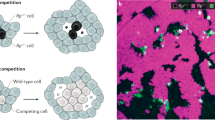
Cells with reduced protein synthesis capacity are eliminated by apoptosis if they are in contact with normal cells but are viable on their own. Conversely, normal cells are eliminated by the presence of abnormally fast-growing cells. This phenomenon, called cell competition, could have evolved as a mechanism to eliminate slow-growing, dysfunctional or aneuploid cells.
-
Cell competition has now been observed in various mosaic tissues. For example, cells deficient in basolateral determinants are eliminated when surrounded by normal cells but not in their own company. It is not known whether the same cell biological mechanism underlies cell competition caused by polarity mismatch and a growth differential.
-
How cells within a population compare their growth rates and how a steep difference leads to the elimination of loser cells are two questions under intense investigation. So far, alternative splicing of the multipass transmembrane protein Flower is the only molecular marker of loser cells (outcompeted cells).
-
Although it is generally assumed that loser cells are killed by a signal emanating from winner cells (faster-growing cells), it has also been suggested that apoptosis could be an indirect consequence of either engulfment by winner cells or delamination caused by differential growth.
-
Recent work has shown that, in Madin–Darby canine kidney (MDCK) cell culture and in the prospective notum of Drosophila melanogaster, tissue crowding leads to the random expulsion of cells from the epithelium and their subsequent apoptosis.
-
Using an established vertex model of epithelial mechanics, the stresses and strains caused by inhomogeneous growth can be predicted. A group of fast-growing cells could cause planar elongation of the surrounding slower-growing cells. This could be the trigger for subsequent delamination and cell elimination. Therefore, strain caused by mechanical stress could contribute to cell competition.
Abstract
When fast-growing cells are confronted with slow-growing cells in a mosaic tissue, the slow-growing cells are often progressively eliminated by apoptosis through a process known as cell competition. The underlying signalling pathways remain unknown, but recent findings have shown that cell crowding within an epithelium leads to the eviction of cells from the epithelial sheet. This suggests that mechanical forces could contribute to cell elimination during cell competition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Plagge, A., Kelsey, G. & Allen, N. D. in Mouse genetics and transgenics, a practical apporach (eds Jackson, I. J. & Abbott, C. M.) (Oxford Univ. Press, 2000).
Oliver, E. R., Saunders, T. L., Tarlé, S. A. & Glaser, T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development 131, 3907–3920 (2004). Provides the first evidence for cell competition in mammals.
Oertel, M., Menthena, A., Dabeva, M. D. & Shafritz, D. A. Cell competition leads to a high level of normal liver reconstitution by transplanted fetal liver stem/progenitor cells. Gastroenterology 130, 507–520 (2006).
Bondar, T. & Medzhitov, R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 6, 309–322 (2010).
Alexander, D. B. et al. Normal cells control the growth of neighboring transformed cells independent of gap junctional communication and SRC activity. Cancer Res. 64, 1347–1358 (2004).
Bignami, M., Rosa, S., La Rocca, S. A., Falcone, G. & Tatò, F. Differential influence of adjacent normal cells on the proliferation of mammalian cells transformed by the viral oncogenes myc, ras and src. Oncogene 2, 509–514 (1988).
Borek, C. & Sachs, L. The difference in contact inhibition of cell replication between normal cells and cells transformed by different carcinogens. Proc. Natl Acad. Sci. USA 56, 1705–1711 (1966).
de Beco, S., Ziosi, M. & Johnston, L. A. New frontiers in cell competition. Dev. Dynam. 241, 831–841 (2012).
Vivarelli, S., Wagstaff, L. & Piddini, E. Cell wars: regulation of cell survival and proliferation by cell competition. Essays Biochem. 53, 69–82 (2012).
Rhiner, C. & Moreno, E. Super competition as a possible mechanism to pioneer precancerous fields. Carcinogenesis 30, 723–728 (2009).
Baker, N. E. & Li, W. Cell competition and its possible relation to cancer. Cancer Res. 68, 5505–5507 (2008).
Hogan, C., Kajita, M., Lawrenson, K. & Fujita, Y. Interactions between normal and transformed epithelial cells: their contributions to tumourigenesis. Int. J. Biochem. Cell Biol. 43, 496–503 (2011).
Morata, G. & Ripoll, P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 42, 211–221 (1975). The first description of cell competition.
Moreno, E., Basler, K. & Morata, G. Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755–759 (2002). Shows that cell death is a landmark of cell competition.
Tamori, Y. & Deng, W.-M. Cell competition and its implications for development and cancer. J. Genet. Genom. 38, 483–495 (2011).
Simpson, P. & Morata, G. Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Dev. Biol. 85, 299–308 (1981).
Ferrus, A. Parameters of mitotic recombination in minute mutants of Drosophila melanogaster. Genetics 79, 589–599 (1975).
Tyler, D. M. & Baker, N. E. Expanded and fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Dev. Biol. 305, 187–201 (2007).
Martín, F. A., Herrera, S. C. & Morata, G. Cell competition, growth and size control in the Drosophila wing imaginal disc. Development 136, 3747–3756 (2009).
Li, W. & Baker, N. E. Engulfment is required for cell competition. Cell 129, 1215–1225 (2007).
Lolo, F.-N., Casas-Tinto, S. & Moreno, E. Cell competition time line: winners kill losers, which are extruded and engulfed by hemocytes. Cell Rep. 2, 526–539 (2012).
Rhiner, C. et al. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Dev. Cell 18, 985–998 (2010).
Portela, M. et al. Drosophila SPARC is a self-protective signal expressed by loser cells during cell competition. Dev. Cell 19, 562–573 (2010).
Xu, T. & Rubin, G. M. The effort to make mosaic analysis a household tool. Development 139, 4501–4503 (2012).
de la Cova, C., Abril, M., Bellosta, P., Gallant, P. & Johnston, L. A. Drosophila myc regulates organ size by inducing cell competition. Cell 117, 107–116 (2004).
Moreno, E. & Basler, K. dMyc transforms cells into super-competitors. Cell 117, 117–129 (2004). Demonstrates, together with reference 25, that Myc is a cell competition factor and discovers super-competition.
Garcia-Bellido, A., Ripoll, P. & Morata, G. Developmental compartmentalisation of the wing disk of Drosophila. Nature: New Biol. 245, 251–253 (1973).
Garcia-Bellido, A., Ripoll, P. & Morata, G. Developmental compartmentalization in the dorsal mesothoracic disc of Drosophila. Dev. Biol. 48, 132–147 (1976).
Marygold, S. The ribosomal protein genes and Minute loci of Drosophila melanogaster. 8, R216 (2007).
Shraiman, B. I. Mechanical feedback as a possible regulator of tissue growth. Proc. Natl Acad. Sci. USA 102, 3318–3323 (2005). Suggests for the first time, that mechanics could influence tissue growth and cell competition.
Moreno, E. Is cell competition relevant to cancer? Nature Rev. Cancer 8, 141–147 (2008).
Baker, N. E. Cell competition. Curr. Biol. 21, R11–R15 (2011).
Lambertsson, A. The minute genes in Drosophila and their molecular functions. Adv. Genet. 38, 69–134 (1998).
Brumby, A. M. & Richardson, H. E. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22, 5769–5779 (2003).
Igaki, T., Pagliarini, R. A. & Xu, T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16, 1139–1146 (2006).
Igaki, T., Pastor-Pareja, J. C., Aonuma, H., Miura, M. & Xu, T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell 16, 458–465 (2009).
Pagliarini, R. A. & Xu, T. A genetic screen in Drosophila for metastatic behavior. Science 302, 1227–1231 (2003).
Vincent, J.-P., Kolahgar, G., Gagliardi, M. & Piddini, E. Steep differences in wingless signaling trigger myc-independent competitive cell interactions. Dev. Cell 21, 366–374 (2011).
Rodrigues, A. B. et al. Activated STAT regulates growth and induces competitive interactions independently of Myc, Yorkie, Wingless and ribosome biogenesis. Development 139, 4051–4061 (2012).
Tamori, Y. & Deng, W.-M. Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Dev. Cell 25, 350–363 (2013).
Huang, J., Wu, S., Barrera, J., Matthews, K. & Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122, 421–434 (2005).
Pan, D. The Hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Halder, G. & Johnson, R. L. Hippo signaling: growth control and beyond. Development 138, 9–22 (2011).
Tyler, D. M., Li, W., Zhuo, N., Pellock, B. & Baker, N. E. Genes affecting cell competition in Drosophila. Genetics 175, 643–657 (2007).
Menéndez, J., Pérez-Garijo, A., Calleja, M. & Morata, G. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc. Natl Acad. Sci. 107, 14651–14656 (2010).
Chen, C.-L., Schroeder, M. C., Kango-Singh, M., Tao, C. & Halder, G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc. Natl Acad. Sci. 109, 484–489 (2012).
Ziosi, M. et al. dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLoS Genet. 6, e100114 (2010).
Tapon, N. et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110, 467–478 (2002).
Udan, R. S., Kango-Singh, M., Nolo, R., Tao, C. & Halder, G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nature Cell Biol. 5, 914–920 (2003).
Lee, K.-P. et al. The Hippo–Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc. Natl Acad. Sci. 107, 8248–8253 (2010).
Song, H. et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl Acad. Sci. 107, 1431–1436 (2010).
Xu, T., Wang, W., Zhang, S., Stewart, R. A. & Yu, W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121, 1053–1063 (1995).
Cho, E. et al. Delineation of a Fat tumor suppressor pathway. Nature Genet. 38, 1142–1150 (2006).
Bennett, F. C. & Harvey, K. F. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr. Biol. 16, 2101–2110 (2006).
Willecke, M. et al. The Fat cadherin acts through the Hippo tumor-suppressor pathway to regulate tissue size. Curr. Biol. 16, 2090–2100 (2006).
Justice, R. W., Zilian, O., Woods, D. F., Noll, M. & Bryant, P. J. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9, 534–546 (1995).
Neto-Silva, R. M., de Beco, S. & Johnston, L. A. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev. Cell 19, 507–520 (2010).
Böhni, R. et al. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97, 865–875 (1999).
Potter, C. J., Tasic, B., Russler, E. V., Liang, L. & Luo, L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell 141, 536–548 (2010).
Froldi, F. et al. The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC Biol. 8, 33 (2010).
Bilder, D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18, 1909–1925 (2004).
Tamori, Y. et al. Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 8, e1000422 (2010).
Humbert, P. O. et al. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 27, 6888–6907 (2008).
Gateff, E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science 200, 1448–1459 (1978).
Bilder, D., Li, M. & Perrimon, N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 (2000).
Garelli, A., Gontijo, A. M., Miguela, V., Caparros, E. & Dominguez, M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science 336, 579–582 (2012).
Colombani, J., Andersen, D. S. & Léopold, P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336, 582–585 (2012).
Norman, M. et al. Loss of Scribble causes cell competition in mammalian cells. J. Cell Sci. 125, 59–66 (2012).
Prober, D. A. & Edgar, B. A. Ras1 promotes cellular growth in the Drosophila wing. Cell 100, 435–446 (2000).
Zhan, L. et al. Deregulation of Scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell 135, 865–878 (2008).
Martín, F. A., Pérez-Garijo, A. & Morata, G. Apoptosis in Drosophila: compensatory proliferation and undead cells. Int. J. Dev. Biol. 53, 1341–1347 (2009).
Rosenblatt, J., Raff, M. C. & Cramer, L. P. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11, 1847–1857 (2001).
Senoo-Matsuda, N. & Johnston, L. A. Soluble factors mediate competitive and cooperative interactions between cells expressing different levels of Drosophila Myc. Proc. Natl Acad. Sci. 104, 18543–18548 (2007).
Petrova, E., López-Gay, J. M., Rhiner, C. & Moreno, E. Flower-deficient mice have reduced susceptibility to skin papilloma formation. Dis. Models Mech. 5, 553–561 (2012).
Ryoo, H. D., Domingos, P. M., Kang, M.-J. & Steller, H. Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 26, 242–252 (2007).
Ohsawa, S. et al. Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev. Cell 20, 315–328 (2011).
Marinari, E. et al. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, 542–545 (2012). Shows that cell crowding leads to live cell delamination in the D. melanogaster notum.
Eisenhoffer, G. T. et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012). Demonstrates that cell crowding leads to live cell extrusion in MDCK epithelia.
Gu, Y., Forostyan, T., Sabbadini, R. & Rosenblatt, J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. J. Cell Biol. 193, 667–676 (2011).
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012).
Bonnet, I. et al. Mechanical state, material properties and continuous description of an epithelial tissue. J. R. Soc., Interface 9, 2614–2623 (2012).
Farhadifar, R., Röper, J.-C., Aigouy, B., Eaton, S. & Jülicher, F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007). First biologist-friendly vertex model of epithelia.
Ranft, J. et al. Fluidization of tissues by cell division and apoptosis. Proc. Natl Acad. Sci. 107, 20863–20868 (2010).
Landsberg, K. P. et al. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr. Biol. 19, 1950–1955 (2009).
Levy, D. L. & Heald, R. Mechanisms of intracellular scaling. Annu. Rev. Cell Dev. Biol. 28, 113–135 (2012).
Teleman, A. A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 425, 13–26 (2010).
Hannezo, E., Prost, J. & Joanny, J.-F. Instabilities of monolayered epithelia: shape and structure of villi and crypts. Phys. Rev. Lett. 107, 078104 (2011).
Mao, Y. et al. Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes Dev. 25, 131–136 (2011).
Aegerter-Wilmsen, T. et al. Exploring the effects of mechanical feedback on epithelial topology. Development 137, 499–506 (2010).
Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nature Rev. Mol. Cell Biol. 7, 265–275 (2006).
Rauzi, M., Lenne, P.-F. & Lecuit, T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114 (2010).
Aigouy, B. et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142, 773–786 (2010).
Ishihara, S. & Sugimura, K. Bayesian inference of force dynamics during morphogenesis. J. Theor. Biol. 313, 201–211 (2012).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010).
Claveria et al. Myc-driven endogenous cell competition in the early mammalian embryo. Nature http://dx.doi.org/10.1038/nature12389 (2013).
Acknowledgements
The authors thank K. Rizzoti and R. Lovell-Badge for discussions about mouse mosaics. J.-P.V. is supported by the Medical Research Council (MRC) and the European Research Council (ERC) (WNTEXPORT),L.A.B.-L. by the Wellcome Trust (082694/z/07/z) and A.G.F. by the Engineering and Physical Sciences Research Council (EPSRC) (EP/I017909/1) and Microsoft Research Cambridge.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Genetic mosaics
-
Tissues comprised of genetically distinct cells. In Drosophila melanogaster, these are typically generated by inducing mitotic recombination in a heterozygous background, thus creating twin clones, one being homozygous wild type and the other homozygous mutant at a given locus.
- Blastocysts
-
Early mammalian embryos composed of about 100 cells. Embryonic stem cells are injected into blastocysts to generate chimaeras before implantation into a surrogate mother.
- Chimaeras
-
Embryos comprising cells of two distinct genotypes. They are usually generated by cell transplantation.
- Apoptosis
-
One of the main processes leading to cell death. It involves the activation of a proteolytic cascade comprising initiator and executioner caspases.
- Aneuploid cells
-
Cells that have lost or gained entire chromosomes or large chromosomal fragments as a consequence of chromosome rearrangements.
- Myc
-
A proto-oncoprotein that controls ribosome biosynthesis, translational activity and other essential cellular activities.
- Contact inhibition
-
A phenomenon whereby cells stop growing as their density increases.
- Imaginal discs
-
Epithelial pouches that grow inside insect larvae and give rise to most adult structures during metamorphosis. They are extensively used to study pattern formation and growth control.
- Apical–basal determinants
-
Proteins that ensure apical–basal polarity. Apical determinants such as atypical protein kinase A and basolateral determinants such as Scribble or Lethal giant larvae typically oppose each other's activity thus ensuring the partitioning of distinct domains.
- Madin–Darby canine kidney cells
-
(MDCK cells). The best-characterized epithelial cell line.
- Neoplastic overgrowth
-
Overgrowth that is accompanied by loss of polarity and general disorganisation of the tissue.
- Hyperplastic overgrowth
-
Overgrowth that maintains cell polarity and tissue integrity. It is often accompanied by tissue folds.
- Caspase
-
A class of proteases that initiate or execute apoptosis.
- JUN N-terminal kinase
-
(JNK). The key mediator of a kinase cascade that is often activated by stress. Depending on the context, it can trigger cell migration or apoptosis.
- hid
-
A gene encoding one of the five pro-apoptotic proteins in Drosophila melanogaster. It inhibits the activity of DIAP1 (D. melanogaster inhibitor of apoptosis 1), which itself inhibits caspases.
- Epithelial-to-mesenchymal transition
-
(EMT). A process whereby epithelial cells lose their epithelial characteristics (for example, polarity), detach from the epithelium and become migratory.
- PTEN
-
A negative regulator of insulin signalling. Loss of PTEN is associated with numerous cancers.
- Tuberous sclerosis complex 1
-
(TSC1). A tumour suppressor that negatively regulates insulin signalling.
- PI3K
-
A key mediator of insulin signalling. Upon activation of the insulin receptor, it converts phosphatidylinositol-4,5-bisphosphate to phosphatidylinositol-3,4,5-triphosphate (PtdIns(3,4,5)P3), an activity that is antagonized by PTEN. PtdIns(3,4,5)P3 activates AKT, which in turn triggers further downstream events.
- Adherens junctions
-
Molecular complex that mediates cell–cell adhesion in epithelia. They are typically organized in belts that surround every cell near the apical side.
Rights and permissions
About this article
Cite this article
Vincent, JP., Fletcher, A. & Baena-Lopez, L. Mechanisms and mechanics of cell competition in epithelia. Nat Rev Mol Cell Biol 14, 581–591 (2013). https://doi.org/10.1038/nrm3639
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3639
This article is cited by
-
Learning biophysical determinants of cell fate with deep neural networks
Nature Machine Intelligence (2022)
-
Matrix mechanics regulates epithelial defence against cancer by tuning dynamic localization of filamin
Nature Communications (2022)
-
Multiple functions of the scaffold protein Discs large 5 in the control of growth, cell polarity and cell adhesion in Drosophila melanogaster
BMC Developmental Biology (2020)
-
Outcompeting cancer
Nature Reviews Cancer (2020)
-
The COX-2/PGE2 pathway suppresses apical elimination of RasV12-transformed cells from epithelia
Communications Biology (2020)