Abstract
Cocaine-associated environmental cues sustain relapse vulnerability by reactivating long-lasting memories of cocaine reward. During periods of abstinence, responding to cocaine cues can time-dependently intensify a phenomenon referred to as ‘incubation of cocaine craving’. Here, we investigated the role of the extracellular matrix protein brevican in recent (1 day after training) and remote (3 weeks after training) expression of cocaine conditioned place preference (CPP). Wild-type and Brevican heterozygous knock-out mice, which express brevican at ~50% of wild-type levels, received three cocaine–context pairings using a relatively low dose of cocaine (5 mg/kg). In a drug-free CPP test, heterozygous mice showed enhanced preference for the cocaine-associated context at the remote time point compared with the recent time point. This progressive increase was not observed in wild-type mice and it did not generalize to contextual-fear memory. Virally mediated overexpression of brevican levels in the hippocampus, but not medial prefrontal cortex, of heterozygous mice prevented the progressive increase in cocaine CPP, but only when overexpression was induced before conditioning. Post-conditioning overexpression of brevican did not affect remote cocaine CPP, suggesting that brevican limited the increase in remote CPP by altering neuro-adaptive mechanisms during cocaine conditioning. We provide causal evidence that hippocampal brevican levels control time-dependent enhancement of cocaine CPP during abstinence, pointing to a novel substrate that regulates incubation of responding to cocaine-associated cues.
Similar content being viewed by others
Introduction
The brain extracellular matrix (ECM) has a critical role in the development of neuronal circuitry and adult neural plasticity (Dityatev and Schachner, 2003; Gundelfinger et al, 2010). Genetic alterations or environmental stimuli that affect the composition of the ECM are thought to contribute to neurodegenerative and psychiatric disorders, including drug addiction (Lubbers et al, 2014a; Vegh et al, 2014). The ECM consists mainly of deposits of hyaluronic acid, glycoproteins and proteoglycans in the extracellular space (Dityatev and Schachner, 2003). Depending on its localization, eg, diffuse, perisynaptic or in perineuronal nets (PNNs), and its molecular composition, the ECM has been shown to either restrict or facilitate neural plasticity processes, including synaptogenesis and synaptic strength (Frischknecht et al, 2009; Pizzorusso et al, 2002). See Dityatev and Schachner (2003) for a review on interactions of ECM molecules with neuronal targets. These processes are also affected by exposure to drugs of abuse and are thought to mediate the persistent nature of drug addiction (Kalivas and O’Brien, 2008; Luscher and Malenka, 2011; Robinson and Kolb, 2004; Van den Oever et al, 2012).
In support of a role of the ECM in addiction, changes in levels of ECM remodelling enzymes, such as matrix metalloproteinases (MMPs), have been observed in post-mortem hippocampal tissue of human cocaine addicts (Mash et al, 2007). Animal models confirm changes in MMP expression following exposure to drugs of abuse and their involvement in drug-associative memories and relapse to drug seeking (Brown et al, 2007; Smith et al, 2014; Van den Oever et al, 2010). Much less is known about the contribution of structural ECM components to addictive behavior. Brevican, a central nervous system specific structural ECM component and constituent of PNNs (Seidenbecher et al, 1995), has been implicated in a diverse set of brain processes, including glioma invasion, Alzheimer’s disease, lesion-induced plasticity, and synaptic plasticity (for a review on brevican functions, see Frischknecht and Seidenbecher, 2012). Notably, brevican has a critical function in maintenance of long-term synaptic potentiation (Brakebusch et al, 2002), the widely acknowledged cellular substrate of memory (Malinow and Malenka, 2002; Nabavi et al, 2014). However, the specific role of brevican in conditioned behavior, and in particular the contribution of brevican to the formation and expression of learned associations in drug addiction, is poorly understood.
Here, we investigated the effect of reduced brevican expression on responding to cocaine-conditioned contextual cues using heterozygous brevican knockout (Bcan+/−) mice and a cocaine CPP paradigm. One day after conditioning with a relatively low dose of cocaine (5 mg/kg), Bcan+/− mice showed normal expression of cocaine CPP, however, CPP was enhanced in these mice when it was assessed 3 weeks after conditioning. A progressive increase in response to cocaine-associated cues during periods of abstinence is a well-established phenomenon in rodent paradigms of extended access to cocaine self-administration (Grimm et al, 2001; Lu et al, 2004), referred to as ‘incubation of cocaine craving’. Moreover, we previously observed this phenomenon with cocaine CPP in wild-type mice using a higher conditioning dose of cocaine (15 mg/kg; Van den Oever et al, 2013) and it has been reported with morphine CPP (Li et al, 2008). Interestingly, the time-dependent enhancement of cocaine CPP in Bcan+/− mice was prevented by virally mediated overexpression of brevican in the dorsal hippocampus (dHPC), but not the medial prefrontal cortex (mPFC). Our data reveal a hitherto undiscovered critical contribution of hippocampal brevican expression to the development of a progressive increase (incubation) in cocaine-conditioned behavior.
Materials and methods
Animals
Bcan+/− mice (Brakebusch et al, 2002) were bred on a C57Bl/6J (Charles River) genetic background. Male Bcan+/− mice and wild-type (Bcan+/+) littermates (>8 weeks old) were individually housed on a 12-h day/night cycle (lights on at 0700 h) in temperature and humidity-controlled rooms. Food and water were available ad libitum. All the experimental procedures were approved by the animal research committee of the VU University Amsterdam.
Immunoblotting
Mice were killed by cervical dislocation. The dorsal half of the hippocampus was dissected from fresh brains and stored at −80 °C (Rao-Ruiz et al, 2011). For the mPFC, brains were removed, rapidly frozen, and the area was dissected freehand from ~1 mm coronal sections in a cryostat. Synaptosomal fractions were prepared as described previously (Lubbers et al, 2014b). After homogenization, 5% of the total volume was collected as total homogenate. Protein samples were treated with 0.2 U/ml chondroitinase ABC (Sigma-Aldrich) for 90 min at 37 °C and SDS-PAGE and immunoblotting were performed and analyzed as previously described (Van den Oever et al, 2008, 2010). Proteins were probed with rabbit anti-brevican (1 : 2000; produced by CIS) or mouse anti-tenascin-R (1 : 2000; P.Glia Cat# pTN-R, RRID:AB_2315473) and alkaline phosphatase or horseradish peroxidase-conjugated secondary antibodies (1 : 10 000; Dako Cat# D0486, D0487, P0447, P0446).
Immunohistochemistry
Immunostainings were performed as described previously (Van den Oever et al, 2013). Mice were transcardially perfused with PBS (pH 7.4) followed by ice-cold 4% paraformaldehyde in PBS. Brains were post-fixed overnight, transferred to 30% sucrose in PBS and allowed to saturate with sucrose for at least 3 days. Brains were then frozen, sliced in 35 μm coronal sections using a cryostat and kept in PBS with 0.02% sodium azide at 4 °C until further use. For visualization of proteins of interest, sections were washed with PBS, blocked with blocking solution (5% goat serum, 2.5% BSA, and 0.2% Triton X-100 in PBS, pH 7.4), and incubated with one or more of the following primary antibodies: guinea pig anti-brevican (1 : 4000; produced by CIS), rabbit anti-parvalbumin (1 : 2000; Swant, Cat# PV 25, RRID:AB_10000344), rabbit anti-GFAP (1 : 2000; Dako, Cat# Z0334, RRID:AB_2314535) in blocking solution overnight at 4 °C. After washing in PBS, sections were incubated with secondary antibodies (goat anti-guinea pig Alexa 568 (1 : 400; Life Technologies, Cat# A11075, RRID:AB_10563254) and donkey anti-rabbit Alexa 647 (1 : 400; Jackson ImmunoResearch Laboratories, Cat# 711-605-152, RRID:AB_2492288), and/or with fluorescein-labeled wisteria floribunda agglutinin (WFA; 1 : 400; Vector Laboratories Cat# FL-1351, RRID:AB_2336875) or Neurotrace 500/525 green fluorescent Nissl (Life Technologies, Cat# N-21480) in blocking solution at RT. Sections were washed in PBS and mounted using 0.2% gelatin in PBS. Images were analyzed by confocal microscopy (LSM 510; Zeiss). For quantification, z-stacks were generated of four (mPFC) or two (dHPC) brain sections per mouse. Neuronal cell bodies stained with green fluorescent Nissl were distinguished from other cell types based on size and intensity.
Cocaine CPP
CPP was performed as described previously using an unbiased, counterbalanced protocol (Van den Oever et al, 2013). Briefly, initial preference for the three compartments of the CPP apparatus was determined in a 10-min pre-conditioning test (pre-test). During three consecutive conditioning days, animals received an i.p. saline injection (morning) or cocaine (5 mg/kg; afternoon; OPG, Utrecht, The Netherlands) before being restricted to one of the outer two compartments for 15 min. The two compartments were counterbalanced based on preference during the pre-test, such that on average all groups did not have a preference for one of the compartments before conditioning (Supplementary Table S1). At days 1 and 21 after conditioning, preference for the cocaine compartment was determined in a drug-free test by allowing animals free access to all the compartments for 5 min (this short test duration minimized within and between session extinction (Van den Oever et al, 2013)). Activity and time spent in each compartment was recorded and analyzed using Ethovision Pro (Noldus). In graphs, activity represents the averaged activity over three conditioning days for saline and cocaine sessions. Preference scores represent time spent in the cocaine-paired compartment minus time spent in the saline-paired compartment.
Contextual Fear Conditioning (FC)
Contextual FC was performed as described previously (Rao-Ruiz et al, 2011), with small modifications. In short, conditioning and testing was performed in a Plexiglas chamber with a stainless steel grid floor (Ugo Basil). For conditioning, mice were placed in the chamber for a period of 180 s, followed by a single foot shock (0.7 mA, 2 s) and a 30-s post-shock period before being placed back into their home cage. At days 1 and 21 after training, animals were returned to the fear-conditioning apparatus for 180 s. Data were recorded and analyzed using Ethovision XT (Noldus). Freezing was defined as a lack of movement except respiration for at least 1.5 s.
Elevated Plus Maze
The elevated plus maze (EPM) consisted of two open arms and two closed arms that were shielded by side and end walls. The arms were connected by a central open area. To test for general anxiety, mice were allowed to explore the arms of the EPM for 10 min. Data were recorded and analyzed using Ethovision Pro (Loos et al, 2009). Anxiety measured in the EPM is presented as the percentage of time spent and entries in the open arms ((time/entries in opens arms divided by the sum of time/entries in open+closed arms) × 100).
AAV2 Vectors and Intracranial Micro-Injection
The full-length coding sequence of the Bcan gene was amplified from mouse brain cDNA, complemented with a C-terminal Myc tag sequence and cloned into a pTRCGW plasmid (AAV-Bcan). AAV serotype 2 (AAV2) particles that contained the CMV minimal promoter and the coding gene sequence of Bcan or green fluorescent protein (AAV-GFP) were produced and purified using a two-plasmid cross-packaging system as described previously (Loos et al, 2014). For intracranial injections, mice were anesthetized with isoflurane, received 0.1 mg/kg Temgesic (RB Pharmaceuticals, UK) and were mounted in a stereotactic frame. Viral vectors (1.0 μl AAV-Bcan (titer: 1.4 × 1012) or 0.5 μl AAV-GFP (titer: 1.3 × 1012); volumes were adjusted for spread of expression) were bilaterally injected into the dorsal region of the mPFC (AP +2.0 mm; ML ±0.45 mm; DV −2.2 mm; relative to Bregma) or dHPC (anteroposterior −1.6 mm; mediolateral ±1.1 mm; dorsoventral −1.7 mm relative to Bregma) at an injection rate of 0.1 μl/min followed by an additional 5 min to allow diffusion (Van den Oever et al, 2013). The mice remained in their home cage for 4 weeks until the start of CPP or until a remote CPP test. The mice were perfused within 1 week after the last CPP or FC test to verify the placement of AAV injections and to confirm brevican overexpression in the dorsal mPFC and dHPC. Mice with virus misplacements (mPFC: AAV-Bcan n=2; dHPC: AAV-GFP n=1) were excluded from further analysis.
Statistical Analysis
Protein expression differences were analyzed using a Student’s t-test or two-way ANOVA with factors genotype and virus. Locomotor activity, preference scores of CPP experiments and freezing levels of FC experiments were analyzed using a mixed-model repeated-measures ANOVA with treatment (saline vs cocaine) and conditioning day or time point (recent vs remote) as within-subject factor and genotype (Bcan+/+ vs Bcan+/−) or virus (AAV-GFP vs AAV-Bcan) as between-subject factor. In case ANOVA’s revealed significant interactions, data were subjected to Bonferroni post hoc analysis, as applicable. In addition, preference for the cocaine context was examined by analyzing the time spent in the saline and cocaine context within each group for each test using a paired Student’s t-tests (Supplementary Table S1). EPM data were analyzed using a Student’s t-test. For immunohistochemical analyses, the chance of co-localization of Bcan+ cells and PV+ (or PNN+) cells was calculated by: (Bcan+/Nissl) × (PV+/Nissl) × 100. The observed co-localization was calculated by: (Bcan+ and PV+/Nissl) × 100.
Results
Brevican Expression in mPFC and dHPC of Bcan+/− Mice
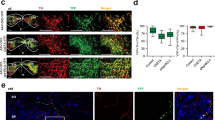
To assess whether reduced brevican levels affect acquisition and expression of cocaine-associated memory, we performed CPP using wild-type (Bcan+/+) mice and heterozygous brevican knockout (Bcan+/−) mice. Bcan+/− mice were used in this study to enable a controlled level of reduced brevican expression, similar to decreased brevican expression after heroin self-administration (Van den Oever et al, 2010), and to avoid gross morphological effects of homozygous brevican knockout on the structure of PNNs (Brakebusch et al, 2002). First, we determined whether brevican expression was reduced in Bcan+/− mice by immunoblotting total protein homogenates of the mPFC and dHPC (Figure 1a–d), regions that have a critical role in cocaine CPP (Meyers et al, 2006; Tzschentke and Schmidt, 1999). Compared with Bcan+/+ mice, Bcan+/− mice showed a significant, 2.4-fold reduction of the 145 kDa brevican isoform (t10=5.7, p<0.001), 1.8-fold reduction of the 130 kDa isoform and 3.1 fold reduction of the 80 kDa isoform (t10=5.9, p<0.001), (t10=2.9, p=0.016) in the mPFC. In the dHPC, we detected a 1.6-fold reduction in 145 kDa (t10=7.9, p<0.001), 2.4-fold reduction in 130 kDa (t10=7.6, p<0.001) and 2.2-fold reduction in 80 kDa (t10=7.4, p<0.001) isoforms in Bcan+/− mice. We next determined whether reduced brevican expression affected expression of tenascin-R (Figure 1a–d), a glycoprotein that co-localizes with brevican in perisynaptic matrix and PNNs (Hagihara et al, 1999; Van den Oever et al, 2010) and directly interacts with brevican (Aspberg et al, 1997). Levels of tenascin-R 160 and 180 kDa isoforms did not differ between Bcan+/+ and Bcan+/− mice in mPFC and dHPC, suggesting that the decrease in brevican expression in Bcan+/− mice was not accompanied by a reduction in other structural components of the ECM. Moreover, in the mPFC and dHPC of Bcan+/− mice, we observed no gross abnormalities in the morphology and intensity of PNNs (Figure 1e), a highly condensed specialization of the ECM (Dityatev and Schachner, 2003). Thus, we found that brevican expression was ~50% decreased in Bcan+/− mice, but levels of an interacting ECM protein and the structure of PNNs appeared unaffected.
Reduced brevican protein expression in Bcan+/− mice. (a–d) Protein levels of brevican (Bcan) and tenascin-R (TnR) isoforms in Bcan+/− mice and Bcan+/+ littermates in total homogenates of the mPFC (a, b; n=6 per group) and dHPC (c, d; n=6 per group). *p<0.05 compared with Bcan+/+. (e) Representative example of PNN structure in the mPFC of Bcan+/+ and Bcan+/− mice. No gross abnormalities were observed in the morphology and intensity of PNNs in the mPFC and dHPC. Scale bar: 10 μm.
Effect of Reduced Brevican Expression on Cocaine CPP Memory
Bcan+/+ and Bcan+/− mice were trained to associate one of two contexts of a CPP apparatus with the rewarding effect of 5 mg/kg cocaine (Figure 2a–c; Van den Oever et al, 2013), a low dose that induces CPP, but without effect on locomotor activity (Supplementary Figure S1). Importantly, this dose allowed bidirectional assessment of cocaine CPP performance in Bcan+/− mice, as it did not evoke a floor or ceiling effect in Bcan+/+ mice. In contrast to Bcan+/+ mice, Bcan+/− mice showed increased locomotor behavior in cocaine compared with saline conditioning sessions (Figure 2b). Repeated-measures ANOVA revealed a significant main effect of treatment (F1,22=9.5, p=0.005) and a genotype × treatment interaction (F1,22=4.4, p=0.048), with post hoc analysis confirming a significant difference between the cocaine and saline sessions of Bcan+/− mice (p=0.030). The cocaine sessions of Bcan+/+ mice did not differ from saline sessions. Although overall Bcan+/− mice showed higher sensitivity to the locomotor enhancing effects of cocaine, ANOVA did not reveal a significant treatment × conditioning day × genotype interaction, indicating that Bcan+/− mice did not develop an enhanced sensitized response to cocaine over conditioning days compared with Bcan+/+ mice. Next, we determined whether reduced brevican levels affected expression of cocaine-CPP in a drug-free test at two time points: at day 1 (recent) and day 21 (remote) after conditioning. Both groups showed similar preference for the cocaine context on day 1, however, preference for the cocaine context robustly increased on day 21 specifically in the Bcan+/− group (Figure 2c). Repeated-measures ANOVA showed a significant effect of time point (F1,21=20.4, p<0.001) and genotype × time-point interaction (F1,21=5.5, p=0.029). Post hoc analysis demonstrated a significant difference between the preference score of Bcan+/− mice at the remote test compared with their recent test (p=0.002) and with the recent test of Bcan+/+ mice (p=0.043).
Reduced brevican expression induced a progressive increase in cocaine CPP. (a) Timeline of CPP and FC. Recent and remote CPP and FC memory was assessed at day 1 and 21, respectively, after conditioning. (b) Increased locomotor activity in Bcan+/− mice during cocaine-conditioning sessions (Bcan+/+ n=12; Bcan+/− n=12). S, saline session; c, cocaine session. *p=0.030 cocaine compared with saline sessions of Bcan+/− mice. (c) Remote CPP expression was significantly increased in Bcan+/−, but not Bcan+/+, mice (n=12 per group). *p<0.05 compared with recent tests. (d) Contextual FC memory expression did not differ between recent and remote time points for both groups (Bcan+/+ n=14; Bcan+/− n=11). (e) Bcan+/+ and Bcan+/− mice did not differ in EPM performance (n=12 per group). All the graphs represent mean±SEM.
We then asked whether the time-dependent CPP enhancement in Bcan+/− mice generalized to other forms of contextual memory. To test this, we used a contextual FC paradigm allowing analysis of responding to contextual cues associated with an aversive stimulus (foot shock) in independent groups of Bcan+/+ and Bcan+/− mice (Figure 2d). After conditioning, both groups showed robust freezing at the recent and remote time point, but no significant effect of time point (F1,23=2.8, p=0.10), nor genotype × time-point interaction (F1,23<0.01, p=0.96). Furthermore, we determined whether CPP performance might have been influenced by a difference in anxiety states between Bcan+/+ and Bcan+/− mice using the elevated plus maze (EPM; Figure 2e). Groups did not differ in the percentage of time spent in the open arms of the EPM (p=0.52) and in the percentage of open arm entries (p=0.66). Thus, mice with reduced brevican expression showed an enhanced response to acute cocaine and incubation of cocaine CPP, with the latter not generalizing to contextual-fear memory and likely not mediated by a difference in anxiety levels.
Overexpression of Brevican in the mPFC
To assess through which brain region reduced brevican protein exerted its effect on cocaine CPP, we generated AAV2 encoding the full-length brevican gene under control of the CMV promoter (AAV-Bcan). On the basis of our previous findings (Van den Oever et al, 2010), we first micro-injected AAV-Bcan or AAV-GFP (control) in the mPFC (aimed at the prelimbic region) of Bcan+/− mice and used an immunohistochemical approach to examine the anatomical distribution of virally overexpressed brevican (Figure 3a) 4 weeks post surgery. Using an antibody against brevican, we observed robust virally expressed brevican (Bcan+) in 23% of neurons (stained with Nissl) in the dorsal region of the mPFC of mice that received AAV-Bcan (Figure 3c), whereas no Bcan+ cells were found in mice that received AAV-GFP. Note that very little endogenous brevican expression was detected in Bcan+/− mice with the current immunostaining conditions. However, AAV-Bcan induced robust extracellular brevican expression in addition to intracellular localization of brevican (Figure 3a and b). Notably, AAV-Bcan was not expressed by astrocytes as Bcan+ and GFAP+ (astrocyte marker) populations showed no overlap (Supplementary Figure S2). In the mPFC, brevican is highly expressed in PNNs that enwrap parvalbumin-expressing inhibitory interneurons (Van den Oever et al, 2010). Therefore, we determined whether Bcan+ co-localized with parvalbumin (PV+) neurons (Figure 3b). A majority (71%) of the PV+ cell population in the mPFC co-localized with Bcan+ cells (Figure 3c). Next, we assessed whether the observed co-localization of Bcan+ and PV+ cells in the total neuronal population differed from chance level (based on the percentage of Bcan+ cells and PV+ cells in the total population). Interestingly, Bcan+ and PV+ co-localized above chance level (t6=−5.5, p=0.002; Figure 3d). As the percentage of PV+ neurons was unaffected by AAV-Bcan in the mPFC (Figure 3e), this indicated that PV+ cells preferentially overexpressed brevican.
Virally mediated overexpression of brevican in the mPFC of Bcan+/− mice. (a) Representative example of brevican expression (Bcan; red), Nissl (green) and PV (blue) in the mPFC of Bcan+/− mice following micro-injection of AAV-GFP (n=4) or AAV-Bcan (n=7). Dashed lines indicate the prelimbic cortex (PL), anterior cingulate cortex (ACC) and forceps minor of the corpus callosum (fmi). Scale bar: 500 μm. (b) Co-localization of brevican overexpressing neurons (Bcan+) and PV+ cells (arrowheads). Scale bar: 20 μm. (c) In mice that received AAV-Bcan, 22.6% of mPFC neurons (Nissl+) were Bcan+, whereas 70% of PV+ cells were Bcan+. (d) Co-localization of Bcan+ and PV+ cells in the total population of Nissl+ neurons exceeded chance level. *p=0.002. (e) AAV-Bcan did not affect the percentage of PV+ cells in mPFC. All the graphs represent mean±SEM. (f–i) Immunoblotting of the mPFC of Bcan+/− mice that received AAV-Bcan (n=4) or AAV-GFP (n=4) confirmed overexpression of Bcan, but not TnR, isoforms, in total homogenates (f and g) and synaptosomal fractions (h and i) *p<0.001 compared with AAV-GFP.
Remodelling of the ECM at perisynaptic sites is thought to contribute to mechanisms of synaptic plasticity and addictive behavior (Smith et al, 2014; Van den Oever et al, 2010). Therefore, we determined whether infusion of AAV-Bcan in the mPFC resulted in increased brevican levels in total protein homogenates and synaptosome-enriched fractions of the mPFC by immunoblotting (Figure 3f–i) 4 weeks post surgery. In both the protein fractions, we detected a robust and significant increase in the 145 kDa brevican isoform (total: t6=−4.4, p=0.004; synaptosomes: t6=−3.7, p=0.010), and a trend for upregulation of the 80 kDa isoform (total: p=0.10; synaptosomes: p=0.10), again without affecting expression of tenascin-R isoforms. Expression of the 130 kDa brevican isoform was robustly increased by AAV-Bcan in total homogenates and synaptosomal fractions (see Figure 3f and h). However, we could not quantify the relative difference of the 130 kDa isoform between groups as the intensity of this band was too low to reliably measure in protein samples of Bcan+/− mice that received AAV-GFP.
Then, we set out to determine the effect of overexpression of brevican levels in the mPFC of Bcan+/− mice on cocaine CPP. Bcan+/− mice received AAV-Bcan or AAV-GFP in the mPFC and 4 weeks later underwent CPP conditioning and preference tests at recent and remote time points (Figure 4a). Although mice that received AAV-Bcan showed a reduced increase in locomotor activity upon cocaine exposure compared with control Bcan+/− mice (Figure 4b), ANOVA only reached significance for treatment (F1,19=6.7, p=0.019), a trend for virus × treatment (F1,19=2.9, p=0.10) and no significant virus × treatment × conditioning day interaction. Moreover, AAV-Bcan did not affect CPP expression as both groups showed enhanced remote compared with recent cocaine CPP scores (Figure 4c). We found a significant main effect of time point (F1,19=22.4, p<0.001), but no virus × time-point interaction (F1,19=0.3, p=0.59). Hence, although a trend was found on reduced sensitivity to the locomotor enhancing effects of cocaine after overexpression of brevican expression in the mPFC of Bcan+/− mice, AAV-Bcan did not prevent incubation of cocaine CPP.
Overexpression of brevican in the mPFC did not affect cocaine CPP. (a) Bcan+/− mice received AAV-GFP (n=10) or AAV-Bcan (n=11) in the mPFC 4 weeks before CPP training. (b) Bcan+/− groups showed enhanced activity during cocaine sessions, but no significant differences between groups were observed. S, saline session; c, cocaine session. (c) Brevican overexpression did not affect preference scores in Bcan+/− mice as both the groups demonstrated increased expression of remote compared with recent cocaine CPP. All the graphs represent mean±SEM.
Overexpression of Brevican in the dHPC
We continued to explore which brain region mediated the time-dependent enhancement of CPP in Bcan+/− mice by studying the effect of AAV-Bcan infusion in the dHPC, a region that is implicated in acquisition and expression of cocaine CPP memory (Meyers et al, 2006). Histological analysis showed that overexpression of brevican in Bcan+/− mice was largely restricted to the CA1 and CA2 region of the dHPC (Figure 5a), and more specifically to the pyramidal cell layer of these regions. We found that ~70% of neurons in the dHPC co-localized with Bcan+ cells (Figure 5d), which was substantially more than in the mPFC (23%; Figure 3c). In the dHPC, 71% of PV+ cells co-localized with Bcan+ cells (Figure 5b and d), however, co-localization of Bcan+ and PV+ did not exceed chance level (p=0.79; Figure 5e). Next, we determined whether Bcan+ cells co-localized with WFA+ PNNs in the dHPC (Figure 5c). Surprisingly, compared with PV cells and the total neuronal population, we found that a slightly lower percentage (53%±11.5) of PNN+ cells co-localized with Bcan+ cells, however, this did not differ significantly from chance level (p=0.22; Figure 5f). Hence, virally expressed brevican was equally distributed over PV cells and non-PV (presumably pyramidal) cells and was not preferentially targeted to PNN-enwrapped neurons in the dHPC.
Virally mediated overexpression of brevican in the dHPC. (a) Representative example of AAV-Bcan (red) and PV (blue) expression in dHPC. Bcan overexpression was largely confined to CA1 and CA2 pyramidal cell layer. Nissl (green) indicates the outline of the dHPC. Scale bar: 500 μm. (b) Co-localization of Bcan+ and PV+ cells in dHPC (arrowheads indicate PV+ cells). Scale bar: 20 μm. (c) Co-localization of Bcan+ cells and WFA+ PNNs in dHPC (arrowheads indicate PNNs). Scale bar: 20 μm. (d) A similar percentage (~70%) of the total neuronal population and PV+ population co-localized with Bcan+ cells (n=6 mice). (e) PV+ and Bcan+ cells co-localized at chance level. (f) WFA+ and Bcan+ cells co-localized at chance level. (g and h) Immunoblotting of dHPC total homogenates derived from Bcan+/+ and Bcan+/− mice that received AAV-GFP or AAV-Bcan (n=4 per group). ANOVA confirmed a main effect of virus for the 130 kDa isoform, whereas levels of the 145 and 80 kDa isoform differed only between genotypes. Expression of TnR isoforms was unaffected.
We then examined AAV-Bcan induced overexpression of the different brevican isoforms in total homogenates of the dHPC by immunoblotting (Figure 5g and h). For this, we compared Bcan+/+ and Bcan+/− mice that received AAV-GFP or AAV-Bcan in the dHPC. Similar to the mPFC, we detected a robust increase of the 130 kDa isoform in Bcan+/− mice that received AAV-Bcan. ANOVA with factors genotype and virus revealed a significant main effect of virus (F1,9=6.0, p=0.038). We only observed a main effect of genotype for the 145 kDa (F1,9=11.6, p=0.008) and 80 kDa (F1,9=6.6, p=0.030) isoforms. The moderate nonsignificant increase of these isoforms in the Bcan+/−+AAV-Bcan group might be explained by the dissection of the entire dHPC. This included regions that were not infected by our AAVs, such as CA3 and the dentate gyrus, which likely diluted the virally mediated overexpression of brevican in CA1. TnR isoforms did not differ between genotype or as a result of virus expression. Taken together, our histological and biochemical analyses confirmed that AAV-Bcan induced overexpression of brevican in the dHPC of Bcan+/− mice, with the 130 kDa isoform showing the highest relative increase in expression.
Next, mice received AAV-Bcan or AAV-GFP in the dHPC 4 weeks before cocaine CPP training (Figure 6a). Overexpression of brevican in the dHPC of Bcan+/− mice appeared to reduce the enhanced locomotor response to acute cocaine during conditioning, similar to the mPFC (Figure 6b). However, we only observed a main effect of treatment (F1,13=5.2, p=0.039), but no significant treatment × virus nor treatment × conditioning day × virus interaction. Despite the absence of effect on locomotor behavior during conditioning sessions, AAV-Bcan completely prevented the time-dependent increase in preference for the cocaine context (Figure 6c). We detected a significant time point (F1,13=12.4, p=0.004) and time-point × virus interaction (F1,13=5.6, p=0.034). Post hoc analysis confirmed that the remote test preference score of Bcan+/− mice that received AAV-GFP differed significantly from their preference score at the recent test (p=0.001), as well as from the recent test of the AAV-Bcan group (p=0.007), whereas this was not true for the remote test of the AAV-Bcan group. CPP performance at the recent test did not differ between groups, indicating that AAV-Bcan did not affect acquisition of CPP memory, but it impaired subsequent memory incubation.
Pre-conditioning overexpression of brevican in the dHPC impaired incubation of cocaine CPP. (a) Bcan+/− mice received AAV-GFP (n=7) or AAV-Bcan (n=8) in the dHPC 4 weeks before cocaine CPP conditioning. (b) Overall, mice showed enhanced locomotor activity in cocaine sessions, but no significant group differences were observed. S, saline session; c, cocaine session. (c) AAV-Bcan prevented the increase in remote cocaine CPP observed in control Bcan+/− mice. *p<0.01 compared with recent tests. (d) One week after the remote CPP test, mice were subjected to contextual FC. Recent and remote contextual FC memory was not affected by AAV-Bcan. (e) Post-conditioning infusion of AAV-Bcan in dHPC did not affect expression of remote cocaine CPP (AAV-GFP n=8; AAV-Bcan n=8). All the graphs represent mean±SEM.
Similar to cocaine CPP, the dHPC, and in particular the CA1 region has a critical role in acquisition and expression of contextual fear memory (Goshen et al, 2011; Tanaka et al, 2014), but whether changes in dHPC brevican levels affect this memory is unknown. One week after the CPP remote test, the same groups underwent contextual FC (Figure 6d). We did not observe incubation of contextual-fear memory in Bcan+/− mice that received control virus and AAV-Bcan in the dHPC also did not affect expression of recent and remote conditioned-fear memory (time-point: F1,13=2.1, p=0.17; time-point × virus: F1,13=0.2, p=0.68). This further indicates that hippocampal brevican levels do not have a general effect on conditioned responding to contextual cues.
As brevican expression was virally enhanced during both conditioning and expression of cocaine CPP, we next questioned whether incubation of cocaine CPP might be impaired by overexpression of brevican after conditioning. Bcan+/− mice were first conditioned and then received AAV-Bcan or AAV-GFP in the dorsal HPC within 1 week after the last conditioning session (Figure 6e). We assessed expression of remote cocaine CPP 4 weeks after microinjection of AAV vectors (to establish the same delay between surgery and the first CPP test as in the experiments described above) and found that both groups showed similar and robust preference for the cocaine context (p=0.43). Hence, the constraint that virally induced brevican exerted on incubation of cocaine CPP was not mediated through an effect on expression of remote CPP, but rather was mediated already during conditioning.
Discussion
Using a genetic approach to control expression of the structural ECM protein brevican, we found that mice with reduced brevican levels show enhanced locomotor activity to acute cocaine and a progressive increase in expression of cocaine CPP during forced abstinence. Incubation of cocaine CPP was selectively prevented by AAV-mediated overexpression of brevican in the dHPC of Bcan+/− mice, whereas overexpression of brevican in the dorsal mPFC was without effect. Interestingly, overexpression of brevican in the dHPC of Bcan+/− mice was only effective when brevican levels were increased before cocaine–context conditioning. Changes in brevican protein levels did not affect contextual-fear memory and did not appear to affect anxiety levels. This, together with the observation that homozygous Bcan−/− mice show no obvious impairments in anxiety and memory tasks (Brakebusch et al, 2002), indicates that differences in dHPC brevican levels during cocaine conditioning have a critical influence on subsequent incubation of cocaine conditioned behavior without having a general effect on learning and memory performance.
Several types of ECM have been characterized previously, including perisynaptic ECM, which closely enwraps individual synapses, and PNNs, which are net-like structures that surround cell bodies and proximal dendrites of subpopulations of neurons throughout the brain (Carulli et al, 2006; Dityatev and Schachner, 2003; Gundelfinger et al, 2010). In Bcan+/− mice, we observed a ~50% reduction of brevican expression in total protein homogenates, and using our AAV approach, we increased brevican in total homogenates, as well as in synapse-enriched fractions. Hence, brevican levels were likely affected in all forms of ECM specializations, preventing firm conclusions on the precise site of action of plasticity mechanisms that contributed to incubation of cocaine seeking. Remarkably, we observed substantial co-localization of virally induced brevican expression within the PV population of the mPFC, pointing to a yet-unknown mechanism that directs brevican expression to this cell type. In line with our finding is the well-established observation that PNNs predominantly enwrap PV neurons in the cortex and hippocampus (Bruckner et al, 1996; Van den Oever et al, 2010), suggesting a shared molecular process that regulates condensation of structural ECM proteins at PV neurons in the cortex. Changes in PNNs have been shown to affect the firing properties of PV cells, thereby influencing GABAergic control over pyramidal cell activity (Dityatev et al, 2007). Indeed, in the mPFC, degradation of PNNs results in reduced inhibitory input onto pyramidal cells and this impairs acquisition of cocaine CPP memory (Slaker et al, 2015). However, overexpression of brevican in the mPFC of Bcan+/− mice did not prevent incubation of cocaine memory, and virally expressed brevican in the dHPC did not preferentially co-localize with PV cells and PNNs, hinting to a mechanism that does not specifically involve PNNs.
By acting as a physical barrier, perisynaptic ECM restricts short-term synaptic plasticity and lateral diffusion of synaptic membrane proteins, such as AMPA receptors, at postsynaptic sites (Frischknecht et al, 2009). One may speculate that a decrease in perisynaptic brevican levels facilitated short-term synaptic plasticity to encode cocaine–context associations. However, apart from a reduction in brevican expression, we did not observe changes in the levels of tenascin-R isoforms and gross morphology of PNNs was not affected in Bcan+/− mice, arguing against a mechanism involving structural ECM changes. Interestingly, homozygous brevican knockout mice have impaired maintenance of long-term potentiation at hippocampal CA1 synapses, an effect that is also observed when acute hippocampal rat brain slices are incubated with an antibody against brevican (Brakebusch et al, 2002). Acute antibody-mediated disruption of LTP maintenance points to a yet-unidentified brevican-regulated cellular signal transduction process, which may also be (partially) affected in Bcan+/− mice. Our biochemical approach allowed detection of the 145 kDa secreted brevican core protein, a 130 kDa membrane-associated isoform that lacks glycosaminoglycan side chains (Viapiano et al, 2003) and an 80 kDa terminally truncated fragment (Seidenbecher et al, 1995). The 130 kDa brevican variant was substantially increased in Bcan+/− mice that received AAV-Bcan, supporting the possibility of a change in a signal transduction process by this membrane-associated form. Future studies should provide more detailed insight in the cellular and molecular mechanisms that mediate incubation of cocaine-conditioned behavior in Bcan+/− mice.
Our data point to the dHPC as an important node in the circuitry that regulates the development of a progressive increase in conditioned response to cocaine cues during abstinence. Post-conditioning overexpression of brevican in the dHPC did not affect remote cocaine CPP in Bcan+/− mice, suggesting that hippocampal brevican prevents incubation by restricting conditioning mechanisms that allow subsequent incubation of CPP. Although it is well established that the dHPC is critically involved in the retrieval of recent contextual memories (Maren et al, 2013), with time, involvement of cortical regions is thought to become progressively more important for memory retrieval (Frankland and Bontempi, 2005). Our data suggest that reduced dHPC brevican expression during cocaine–context conditioning gate plasticity mechanisms that enable the conditioned response to subsequently incubate over time, potentially by allowing more efficient recruitment or strengthening of (in)direct connections with cortical regions, such as the anterior cingulate cortex or ventral mPFC (Frankland et al, 2004; Koya et al, 2009; Rajasethupathy et al, 2015). In line with this reasoning, cocaine CPP may no longer be influenced by changes in hippocampal brevican levels when cortical regions are playing a more prominent role. Alternatively, altered functioning of the dHPC as a result of a change in brevican expression may impact neuro-adaptive mechanisms in other brain regions that are implicated in the incubation of cocaine craving, such as the nucleus accumbens and amygdala (Conrad et al, 2008; Lu et al, 2005).
We previously found that perisynaptic expression of brevican and other structural ECM components was reduced in the medial prefrontal cortex (mPFC) of rats during abstinence from heroin self-administration (Van den Oever et al, 2010). Recovery of these ECM constituents using a non-selective MMP inhibitor attenuated cue-induced relapse to heroin seeking (Van den Oever et al, 2010). These data pointed to an important role of mPFC structural ECM proteins in conditioned drug seeking, however, selectively restoring brevican levels in the mPFC using a viral vector approach did not affect expression of recent and remote cocaine CPP in the present study. Although the mPFC has been implicated in the acquisition of cocaine CPP memory (Tzschentke and Schmidt, 1999), a selective change in brevican levels within this region might be insufficient to affect the encoding of contextual-cocaine memory, because memory acquisition may more heavily rely on other brain regions, such as the dHPC (Meyers et al, 2006). Moreover, the absence of effect upon overexpression of brevican in the dorsal mPFC is in line with the observation that pharmacological inactivation of the dorsal mPFC does not affect incubation of response to cocaine cues following 30 days of abstinence from cocaine self-administration (Koya et al, 2009).
To conclude, we provide evidence that the hippocampal ECM protein brevican is involved in incubation of cocaine-conditioned behavior. More specifically, we show that changes in brevican levels during formation of cocaine–context associations affect subsequent responding to cocaine-associated cues. This, together with the observation that Bcan+/− mice showed enhanced locomotor activity to acute cocaine, suggests that individuals with a (genetic) predisposition towards reduced brevican expression may be more sensitive to cocaine and may act more vigorously on cocaine cues following prolonged withdrawal periods. Future experiments should provide further insight in the use of the ECM as a therapeutic target for the treatment of cocaine addiction.
Funding and Disclosure
BRL received funding from ZonMw grant 9120909005. MRM is funded by EU MSCA-ITN CognitionNet (FP7-PEOPLE-2013-ITN 607508). RF received funding from Schram Stiftung (T287/21796/2011), and RF and CIS were funded by CRC779/B14. ABS and SS are funded partly by a grant awarded to NeuroBasic Pharmaphenomics consortium, and SS is funded by an NWO-ALW VICI grant (865.14.002). MCvdO was funded by a ZonMw VENI grant (916.12.034) and Hersenstichting Nederland grant (KS 2012(1)-162). All authors declare no conflict of interest.
References
Aspberg A, Miura R, Bourdoulous S, Shimonaka M, Heinegard D, Schachner M et al (1997). The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci USA 94: 10116–10121.
Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H et al (2002). Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol 22: 7417–7427.
Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA (2007). Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem 14: 214–223.
Bruckner G, Hartig W, Kacza J, Seeger J, Welt K, Brauer K (1996). Extracellular matrix organization in various regions of rat brain grey matter. J Neurocytol 25: 333–346.
Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K et al (2006). Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol 494: 559–577.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y et al (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454: 118–121.
Dityatev A, Bruckner G, Dityateva G, Grosche J, Kleene R, Schachner M (2007). Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol 67: 570–588.
Dityatev A, Schachner M (2003). Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci 4: 456–468.
Frankland PW, Bontempi B (2005). The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130.
Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ (2004). The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304: 881–883.
Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED (2009). Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 12: 897–904.
Frischknecht R, Seidenbecher CI (2012). Brevican: a key proteoglycan in the perisynaptic extracellular matrix of the brain. Int J Biochem Cell Biol 44: 1051–1054.
Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C et al (2011). Dynamics of retrieval strategies for remote memories. Cell 147: 678–689.
Grimm JW, Hope BT, Wise RA, Shaham Y (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412: 141–142.
Gundelfinger ED, Frischknecht R, Choquet D, Heine M (2010). Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix. Eur J Neurosci 31: 2156–2165.
Hagihara K, Miura R, Kosaki R, Berglund E, Ranscht B, Yamaguchi Y (1999). Immunohistochemical evidence for the brevican-tenascin-R interaction: colocalization in perineuronal nets suggests a physiological role for the interaction in the adult rat brain. J Comp Neurol 410: 256–264.
Kalivas PW, O’Brien C (2008). Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33: 166–180.
Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y (2009). Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56 (Suppl 1): 177–185.
Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM et al (2008). Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci 28: 13248–13257.
Loos M, Mueller T, Gouwenberg Y, Wijnands R, van der Loo RJ, Birchmeier C et al (2014). Neuregulin-3 in the Mouse Medial Prefrontal Cortex Regulates Impulsive Action. Biol Psychiatry. 76: 648–655.
Loos M, van der Sluis S, Bochdanovits Z, van Zutphen IJ, Pattij T, Stiedl O et al (2009). Activity and impulsive action are controlled by different genetic and environmental factors. Genes Brain Behav 8: 817–828.
Lu L, Grimm JW, Hope BT, Shaham Y (2004). Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47 (Suppl 1): 214–226.
Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y (2005). Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8: 212–219.
Lubbers BR, Smit AB, Spijker S, van den Oever MC (2014a). Neural ECM in addiction, schizophrenia, and mood disorder. Prog Brain Res 214: 263–284.
Lubbers BR, van Mourik Y, Schetters D, Smit AB, De Vries TJ, Spijker S (2014b). Prefrontal gamma-aminobutyric acid type a receptor insertion controls cue-induced relapse to nicotine seeking. Biol Psychiatry 76: 750–758.
Luscher C, Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69: 650–663.
Malinow R, Malenka RC (2002). AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126.
Maren S, Phan KL, Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14: 417–428.
Mash DC, ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J (2007). Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS One 2: e1187.
Meyers RA, Zavala AR, Speer CM, Neisewander JL (2006). Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 120: 401–412.
Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R (2014). Engineering a memory with LTD and LTP. Nature 511: 348–352.
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002). Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298: 1248–1251.
Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY et al (2015). Projections from neocortex mediate top-down control of memory retrieval. Nature 526: 653–659.
Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB et al (2011). Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci 14: 1302–1308.
Robinson TE, Kolb B (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47 (Suppl 1): 33–46.
Seidenbecher CI, Richter K, Rauch U, Fassler R, Garner CC, Gundelfinger ED (1995). Brevican, a chondroitin sulfate proteoglycan of rat brain, occurs as secreted and cell surface glycosylphosphatidylinositol-anchored isoforms. J Biol Chem 270: 27206–27212.
Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J et al (2015). Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci 35: 4190–4202.
Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA et al (2014). Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci 17: 1655–1657.
Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, Wiltgen BJ (2014). Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84: 347–354.
Tzschentke TM, Schmidt WJ (1999). Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci 11: 4099–4109.
Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer AN et al (2008). Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci 11: 1053–1058.
Van den Oever MC, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M et al (2010). Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology 35: 2120–2133.
Van den Oever MC, Rotaru DC, Heinsbroek JA, Gouwenberg Y, Deisseroth K, Stuber GD et al (2013). Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. J Neurosci 33: 18225–18233.
Van den Oever MC, Spijker S, Smit AB (2012). The synaptic pathology of drug addiction. Adv Exp Med Biol 970: 469–491.
Vegh MJ, Heldring CM, Kamphuis W, Hijazi S, Timmerman AJ, Li KW et al (2014). Reducing hippocampal extracellular matrix reverses early memory deficits in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun 2: 76.
Viapiano MS, Matthews RT, Hockfield S (2003). A novel membrane-associated glycovariant of BEHAB/brevican is up-regulated during rat brain development and in a rat model of invasive glioma. J Biol Chem 278: 33239–33247.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Lubbers, B., Matos, M., Horn, A. et al. The Extracellular Matrix Protein Brevican Limits Time-Dependent Enhancement of Cocaine Conditioned Place Preference. Neuropsychopharmacol 41, 1907–1916 (2016). https://doi.org/10.1038/npp.2015.361
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.361
This article is cited by
-
Net gain and loss: influence of natural rewards and drugs of abuse on perineuronal nets
Neuropsychopharmacology (2023)
-
The extracellular matrix and perineuronal nets in memory
Molecular Psychiatry (2022)
-
From stress to depression: development of extracellular matrix-dependent cognitive impairment following social stress
Scientific Reports (2020)
-
Memory strength gates the involvement of a CREB-dependent cortical fear engram in remote memory
Nature Communications (2019)
-
Engram-specific transcriptome profiling of contextual memory consolidation
Nature Communications (2019)