Abstract
The basolateral amygdala (BLA) and lateral orbitofrontal cortex (OFC) are critical elements of the neural circuitry that regulates drug context-induced reinstatement of cocaine-seeking behavior. Given the existence of dense reciprocal anatomical connections between these brain regions, this study tested the hypothesis that serial information processing by the BLA and OFC is necessary for drug context-induced cocaine-seeking behavior. Male Sprague–Dawley rats were trained to lever press for un-signaled cocaine infusions (0.15 mg/infusion, i.v.) in a distinct environment (cocaine-paired context) then underwent extinction training in a different environment (extinction context). During four subsequent test sessions, rats were re-exposed to the cocaine-paired and extinction contexts in order to assess cocaine-seeking behavior (non-reinforced active lever responding). Immediately before each test session, rats received microinfusions of the GABAA/GABAB agonist cocktail, baclofen+muscimol (BM: 1.0/.01 mM), or vehicle unilaterally into the BLA plus the contralateral or ipsilateral OFC, or unilaterally into the OFC alone. Exposure to the previously cocaine-paired context, but not the extinction context, reinstated extinguished cocaine-seeking behavior. BM-induced unilateral OFC inactivation failed to alter this behavior, similar to the effect of unilateral BLA inactivation in our previous study (Fuchs et al, 2007). Conversely, neural inactivation of the BLA plus the contralateral or ipsilateral OFC equally attenuated drug context-induced cocaine seeking without altering food-reinforced instrumental responding, relative to vehicle pretreatment. These findings suggest that the BLA and OFC co-regulate drug context-induced motivation for cocaine either through sequential information processing via intra- and interhemispheric connections or by providing converging input to a downstream brain region.
Similar content being viewed by others
INTRODUCTION
Cocaine addiction is a chronic relapsing disorder characterized by compulsive drug seeking and drug taking even after prolonged abstinence periods. Environmental stimuli repeatedly paired with the effects of cocaine can acquire conditioned rewarding, conditioned reinforcing, and incentive motivational properties through associative learning processes (Crombag et al, 2008; Fuchs et al, 2008a). As a result, drug-paired explicit or contextual stimuli can produce drug craving and relapse in abstinent drug users (Rohsenow et al, 1990; Everitt et al, 1991; Ehrman et al, 1992; Childress et al, 1993; Foltin and Haney, 2000), and reinstate extinguished drug-seeking behavior in laboratory animals (Crombag et al, 2002; Fuchs et al, 2005; Alleweireldt et al, 2006; Fuchs et al, 2008a; Fuchs et al, 2008b; Xie et al, 2010). Drug context-induced relapse to cocaine seeking is regulated by a mesocorticolimbic neural circuitry; hence, understanding the interactions between elements of this neurocircuitry is important from an addiction treatment perspective.
Extensive evidence suggests that the basolateral amygdala (BLA) and lateral orbitofrontal cortex (OFC) regulate drug context-induced cocaine-seeking behavior. In cocaine users, exposure to cocaine-paired stimuli elicits enhanced neural activity in the BLA and OFC, which has been positively correlated with self-reports of cocaine craving (Grant et al, 1996; Childress et al, 1999; London et al, 1999; Kilts et al, 2001). Similarly, in cocaine-experienced rats, re-exposure to drug-paired contexts elicits neural activation in the BLA and OFC concomitant with drug-seeking behavior (Neisewander et al, 2000; Hamlin et al, 2008; Hearing et al, 2008a; Hearing et al, 2008b). Moreover, the functional integrity of the BLA and OFC is necessary for drug context-induced reinstatement of cocaine seeking (See et al, 2001; Kantak et al, 2002; McLaughlin and See 2003; See et al, 2003; Fuchs et al, 2004; Fuchs et al, 2005; Lasseter et al, 2009).
The BLA and OFC may be part of a serial neural circuit, such that sequential information processing by these brain regions may critically contribute to drug context-induced cocaine-seeking behaviors. The BLA and OFC not only receive sensory input from cortical areas, but also share dense direct reciprocal projections and interact via thalamic relays (Ray and Price 1992; Carmichael and Price 1995; Ghashghaei and Barbas 2002; Corbit et al, 2003). Converging lines of evidence suggest that functional interactions between the BLA and OFC are necessary for a variety of goal-directed behaviors (Baxter et al, 2000; Saddoris et al, 2005; Churchwell et al, 2009; Takahashi et al, 2009). In fact, it has been postulated that maladaptive drug-seeking behaviors may reflect cocaine-induced neurophysiological abnormalities in the orbitofrontal–amygdalar circuit (Takahashi et al, 2009). However, no study to date has investigated whether the BLA and OFC functionally interact to direct drug context-induced cocaine seeking or, alternatively, control this behavior independently via parallel circuitries.
This study used a functional disconnection procedure to explore whether the BLA and OFC exhibit sequential information processing to regulate drug context-induced reinstatement of cocaine-seeking behavior. Dense intra- and interhemispheric connections exist between the BLA and OFC (Barbas and Pandya, 1984; Cavada et al, 2000); hence, the functional significance of interactions via both ipsilateral and contralateral projections was investigated. To bilaterally disrupt intrahemispheric neural communication between the BLA and OFC, baclofen+muscimol (BM), a GABA agonist cocktail that suppresses neural activity in cell bodies without affecting fibers of passage (Martin and Ghez, 1999), was infused unilaterally into the BLA plus the contralateral OFC immediately before assessing drug context-induced cocaine-seeking behavior. To bilaterally disrupt interhemispheric communication between the BLA and OFC, additional groups received BM infusions unilaterally into the BLA plus the ipsilateral OFC. Because unilateral manipulation of either the BLA or OFC may alter context-induced cocaine seeking, functional interdependence between the BLA and OFC via intrahemispheric and interhemispheric connections was predicted to manifest as a superadditive effect following the contralateral or ipsilateral manipulation, respectively, relative to the sum of effects observed following unilateral manipulations of the brain regions. Our laboratory has previously verified that unilateral BLA inactivation fails to produce a statistically significant impairment in drug context-induced cocaine seeking (Fuchs et al, 2007). Thus, to test for a superadditive effect, a separate control group received unilateral manipulations of the OFC. Given that intrahemispheric connections between the BLA and OFC have been cited as critical for behavioral control (Churchwell et al, 2009), we predicted that contralateral BLA/OFC functional inactivation would produce greater impairment in drug context-induced cocaine seeking than the ipsilateral manipulation or unilateral functional inactivation of the OFC. To discriminate between impairments in motivation and motor performance, we also assessed the effects of contralateral and ipsilateral BLA/OFC neural inactivation on locomotor and food-reinforced instrumental behaviors.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (n=53; 250–300 g; Charles River, Wilmington, MA, USA) were housed individually in a climate-controlled vivarium on reversed light–dark cycle. Rats received 20–25 g of rat chow per day with water available ad libitum. Animal housing and treatment protocols followed the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, 1996) and were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Food Training
To expedite cocaine self-administration training, all rats (n=53) were trained to lever press on a fixed ratio 1 (FR1) schedule of food reinforcement (45 mg pellets; Noyes, Lancaster, NH, USA) in sound-attenuated operant conditioning chambers (26 × 27 × 27 cm high; Coulbourn Instruments, Allentown, PA, USA) during a 16-h overnight session. Active lever responses resulted in the delivery of one food pellet only; inactive lever responses had no programmed consequences. During food training, contextual stimuli subsequently used for cocaine conditioning were not present.
Surgery
At 48 h after food training, all rats were fully anesthetized using ketamine hydrochloride and xylazine (66.6 and 1.33 mg/kg, i.p., respectively). Chronic indwelling jugular catheters were constructed in house and were surgically implanted into the right jugular vein of a subset of rats (n=35), as described previously (Fuchs et al, 2007). All rats (n=53) were stereotaxically implanted with stainless steel guide cannulae (26 gauge; Plastics One) aimed dorsal to the left or right BLA (−2.7 mm AP, ±5.2 mm ML, −6.7 mm DV, relative to bregma) and/or the left or right OFC (+3.5 mm AP, ±3.0 mm ML, −3.4 DV) using standard procedures. Stainless steel screws and cranioplastic cement secured the guide cannulae to the skull. Stylets (Plastics One) and Tygon caps sealed the guide cannulae and catheter, respectively. To extend catheter patency, the catheters were flushed daily with an antibiotic solution of cefazolin (10.0 mg/ml; Schein Pharmaceuticals, Albuquerque, NM, USA) and heparinized saline (70 U/ml; Baxter Health Care Corp, Deerfield, IL, USA), as described previously (Fuchs et al, 2007). Rats were given a 5-day postoperative recovery period before the start of the experiment. Catheter patency was evaluated periodically using propofol (1 mg/0.1 ml, i.v. Eli Abbott Lab, North Chicago, IL, USA), which produces a rapid loss of muscle tone only when administered intravenously.
Cocaine Self-Administration Training
Cocaine self-administration and extinction training sessions were conducted in operant conditioning chambers configured to one of two unique environmental contexts (context 1, context 2) that differed along visual, auditory, tactile, and olfactory modalities, as described previously (Fuchs et al, 2005; Fuchs et al, 2007; Fuchs et al, 2008b). Rats had no exposure to these contextual stimuli before cocaine self-administration training; these stimuli were presented throughout each session independent of responding.
The rats were randomly assigned to receive daily 2-h cocaine self-administration training sessions in context 1 or 2 during their dark cycle. Responses on one (active) lever were reinforced under an FR1 schedule of cocaine reinforcement (cocaine hydrochloride; 0.15 mg/infusion, equivalent to ∼0.50 mg/kg/infusion; i.v.; NIDA, Research Triangle Park, NC, USA). A 20-s time-out period followed each 2 s infusion during which lever responses were recorded, but had no programmed consequences. Responses on the other (inactive) lever were recorded but had no programmed consequences. Training continued until the rats obtained ⩾10 cocaine infusions/session on at least 10 training days (ie, acquisition criterion).
Extinction Training
After meeting the acquisition criterion, rats received daily 2-h extinction training sessions in the context (context 1 or 2) that distinctly differed from the cocaine self-administration training context. Lever presses were recorded, but had no programmed consequences. Immediately before the behavioral session on extinction day 4, rats were acclimated to the intracranial infusion procedure. To this end, injection cannulae (33 Ga, Plastics One) were inserted into the guide cannulae of the rats to a depth 2 mm below the tip of the guide cannulae, and were left in place for 4 min. No drug was infused. Extinction training consisted of a minimum of seven sessions plus additional sessions, as needed, until the rats reached the extinction criterion (⩽25 active lever presses/session for two consecutive sessions).
Reinstatement Testing
After the rats reached the extinction criterion, cocaine seeking was assessed in the cocaine-paired and extinction contexts during four test sessions (Figure 1). Immediately before testing, rats received microinfusions of the GABAB/GABAA agonist cocktail baclofen+muscimol (BM; 1.0/0.1 mM; 0.5 μl/hemisphere, respectively; pH ∼7.0) or phosphate buffered saline vehicle (VEH; 0.5 μl/hemisphere) either (a) unilaterally into the BLA plus contralateral OFC, (b) unilaterally into the BLA plus ipsilateral OFC, or (c) unilaterally into the OFC. This BM dose was selected on the basis of previous findings, that administration of this dose into the BLA or OFC impairs drug context-induced cocaine-seeking behavior in a brain region-specific manner (Fuchs et al, 2007; Lasseter et al, 2009). The infusions were delivered over 2 min, and the injection cannulae were left in place for 1 min before and 1 min after the infusion. During the test sessions, lever responding had no programmed consequences. Session length was 1 h to allow for repeated testing without significant extinction learning. Both the order of testing in the two contexts and the order of intracranial treatments (BM, VEH) were counterbalanced based on mean active lever responding during the last 3 self-administration training days. Subjects received additional extinction sessions in the extinction context between test sessions until they re-obtained the extinction criterion (see above).
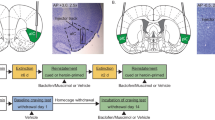
Schematic representation of the timeline for the context-induced reinstatement experiments (a) and instrumental control experiments (b). Arrows on the schematic identify the test sessions when vehicle (VEH) or baclofen plus muscimol (BM) was administered. The order of drug treatment (BM, VEH) and the order of exposure to the two testing contexts (cocaine-paired context, COC CTX; extinction context, EXT CTX) was counterbalanced, where appropriate. Asterisks indicate that the acquisition criterion (⩾10 infusions per session for minimum 10 sessions) and extinction criterion (⩽25 active lever presses per session for two consecutive sessions) had to be met before moving on to the next experimental phase. Hash symbols indicate that the stability criterion had to be met before moving on to the next experimental phase.
Locomotor Activity and Food-Reinforced Instrumental Behavior
Intracranial manipulations can produce motor deficits that impair instrumental performance. Hence, the effects of BM and VEH treatment on general locomotor activity and food-reinforced instrumental behavior were examined.
Locomotor activity was assessed during two 1-h test sessions held 5 days apart, starting 48 h after the last reinstatement test session. Locomotor activity was measured in novel Plexiglas chambers (42 × 20 × 20 cm) equipped with an array of eight photodetectors and light sources. Before testing, rats received intracranial microinfusions of BM or VEH using the infusion procedures and treatment order applied in the reinstatement experiment. A computerized activity system (San Diego Instruments, San Diego, CA) recorded the number of consecutive photobeams interrupted by rats moving in the activity chamber.
Food-reinforced lever pressing behavior was assessed in experimentally naïve rats (n=18). After overnight food training and stereotaxic surgery (described above), the rats received additional daily 2-h food self-administration training sessions in context 1 or 2 until responding stabilized (ie, ⩽20% variability in active lever responding across two consecutive sessions), using previously described methods (Xie et al, 2010). After the stability criterion was reached, two 1-h test sessions were conducted. Immediately before the test sessions, rats received BM or VEH infusions into the BLA and the ipsilateral or contralateral OFC using the infusion procedure described above. The order of intracranial treatments was counterbalanced across the two test sessions based on mean active lever responding during the last two training sessions. During the training and test sessions, active lever presses were reinforced with food pellets (45 mg, Purina) under an FR1 schedule with a 20-s timeout period. Inactive lever presses were recorded, but had no programmed consequences. Between the test sessions, rats received a minimum of two additional food self-administration training sessions to reestablish baseline responding.
Histology
After the last experimental session, rats were overdosed using ketamine hydrochloride and xylazine (66.6 and 1.3 mg/kg i.v. or 199.8 and 3.9 mg/kg i.p., respectively, depending on catheter patency). The brains were dissected out, stored in 10% of formaldehyde solution, and then sectioned at a thickness of 75 μm using a vibratome. The sections were stained using cresyl violet (Kodak, Rochester, NY, USA). Cannula placements were verified using light microscopy and were mapped onto schematics from the rat brain atlas (Paxinos and Watson 1997).
Data Analysis
Only data from rats with correct cannula placements were included in the data analysis. Potential pre-existing differences between the treatment groups in (a) lever responses and cocaine intake during the last 3 days of self-administration training, (b) lever responses during the first 7 days of extinction training, and (c) the number of days needed to reach the extinction criterion were analyzed using mixed factors ANOVAs with surgery condition (contralateral, ipsilateral, and unilateral) and subsequent treatment order (BM first, VEH first) as between-subjects factors and time (day) as the within-subjects factor, where appropriate. The effects of BM and VEH infusions on (a) lever responses during the contextual reinstatement test sessions, (b) the number of photobeam breaks during the locomotor activity tests, and (c) food-reinforced instrumental responding were assessed using mixed factors ANOVAs with surgery condition (ipsilateral and contralateral) as the between-subjects factor and treatment (BM, VEH), testing context (cocaine-paired context, extinction context), time (20 min intervals), and/or lever (active, inactive) as within-subjects factors, when appropriate. Because the variables BLA hemisphere (left, right) and OFC hemisphere (left, right) are not orthogonal, the hemispheric laterality of significant effects was analyzed separately for the contralateral, ipsilateral, and unilateral surgery groups using planned t-tests. Significant main and interaction effects were investigated using simple main effects tests or Tukey post hoc tests. The α was set at 0.05.
RESULTS
Histology
Photomicrographs of representative cannula placements as well as schematics of the distribution of cannula placements are provided in Figure 2. The target brain regions were defined as the basolateral and lateral nuclei of the amygdala (BLA) and the lateral and ventrolateral subregions of the OFC (OFC). High power microscopy confirmed that there was no evidence of abnormal tissue damage (ie, extensive cell loss or gliosis) at the infusion sites. Data from rats with misplaced cannulae (n=9) were excluded from data analysis. For the remaining cocaine-trained rats (n=29), the most ventral point of the cannula tract was correctly located within the target brain regions of the contralateral (right BLA/left OFC, n=4; left BLA/right OFC, n=6), ipsilateral (right BLA/OFC, n=6; left BLA/OFC, n=4), and unilateral groups (right OFC, n=5; left OFC, n=4). For the remaining food-trained rats (n=15), the cannula tract was correctly located within the target brain regions of the contralateral (right BLA/left OFC, n=4; left BLA/right OFC, n=4) and ipsilateral groups (right BLA/OFC, n=4; left BLA/OFC, n=3).
Schematic and photographic representation of injection cannula placements. The arrows on the photomicrographs identify the most ventral point of the infusion cannula tracts on representative cresyl violet-stained sections. The symbols on the schematics (Paxinos and Watson, 1997) represent the most ventral point of the infusion cannula tracts for rats that received unilateral microinfusions into the BLA plus the contralateral OFC (cocaine self-administration: closed circles, food-maintained responding: open circles) or the ipsilateral OFC (cocaine self-administration: filled triangles, food-maintained responding: open triangles), or a unilateral microinfusion into the OFC alone (filled squares). Numbers indicate the distance from bregma in millimeters.
Self-Administration Responding
The groups with cannulae aimed at the contralateral or ipsilateral BLA/OFC or unilaterally at the OFC exhibited stable active lever responding for cocaine reinforcement over the last 3 days of self-administration training, with a within-subjects variability of <10% in daily cocaine intake. There was no difference between these groups in active lever responding (all day and surgery type main effects and interactions, all Fs ⩽1.02, p⩾ 0.41) or inactive lever responding (all day and surgery type main and interaction effects, all Fs ⩽1.14, p ⩾0.34) during the last 3 days of self-administration training. Collapsed across groups, the mean active and inactive lever responding±SEM was 42.92±3.13 and 4.42±1.72, respectively, whereas the mean cocaine intake±SEM was ∼11.55±0.83 mg/kg per session (23.10±1.65 infusions; data not shown). Separate ANOVAs further indicated that there were no pre-existing differences in either lever responding or cocaine intake during the last 3 self-administration training days as a function of surgery condition (contralateral, ipsilateral, and unilateral) or subsequent treatment order (BM first, VEH first; all treatment order main effects and interactions, all Fs ⩽2.39, p⩾0.14), or hemispheric laterality (left, right; all ts ⩽1.61, p⩾0.12).
Extinction Responding
On removal of cocaine reinforcement during extinction training, active and inactive lever responding gradually declined (active lever: day main effect, F(6,156)=24.58, p=0.0001; day 1 >day 2–7, Tukey test, p<0.01; inactive lever: day main effect, F(6,156)=10.10, p=0.0001; day 1 > day 2–7, Tukey test, p<0.05). There was no difference between the contralateral BLA/OFC-cannulated, ipsilateral BLA/OFC-cannulated, and unilateral OFC-cannulated groups in active or inactive lever responding during the first 7 days of extinction training (all surgery type main effects and surgery type X day interactions, all Fs ⩽2.83, p⩾0.08). Separate ANOVAs indicated that there were no differences in active lever responding as a function of subsequent treatment order for either the contralateral BLA/OFC- or ipsilateral BLA/OFC-cannulated groups (all treatment order main effects and treatment order X day interactions, all Fs ⩽0.69, p⩾0.25). The unilateral OFC-cannulated group that subsequently received VEH exhibited more active lever pressing than the group that subsequently received BM (treatment order X day interaction, F(6,42)=0.41, p=0.003; treatment order main effect, F(1,7)=5.60, p=0.050) on the first day of extinction training (VEH >BM day 1; Tukey test, p<0.05), after which no group differences were observed. There was no difference between groups of similar surgery condition in inactive lever responding based on subsequent treatment order (all treatment order main effects and treatment order X day interactions, all Fs ⩽1.78, p⩾0.11). There was also no difference in the mean number of days±SEM required to reach the extinction criterion (7.45±1.61) as a function of surgery condition (contralateral, ipsilateral, and unilateral; F(2,26)=0.22, p=0.81) or subsequent treatment order (all ts<1.90, p>0.10). Similarly, there was no difference between the groups in the mean number of days+SEM needed to re-obtain the extinction criterion between reinstatement test sessions (2.1+0.06). Hence, it is unlikely that pre-existing differences accounted for group differences in reinstatement responding during the subsequent test sessions.
Effects of BLA/OFC Functional Inactivation on Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior
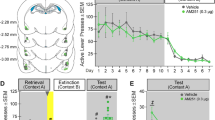
After VEH pretreatment, the contralateral and ipsilateral BLA/OFC-cannulated groups exhibited an increase in non-reinforced active lever responding on exposure to the previously cocaine-paired context relative to responding in the extinction context (Figures 3a and b; context main effect, F(1,18)=50.04, p=0.0001). BM pretreatment impaired active lever responding relative to VEH pretreatment in a context-specific manner after administration into either the contralateral BLA/OFC or the ipsilateral BLA/OFC (treatment X context interaction, F(1,18)=33.51, p=0.0001; treatment main effect, F(1,18)=24.49, p=0.0001; only statistically significant effects are reported). Specifically, independent of surgery condition, BM pretreatment attenuated active lever responding relative to VEH pretreatment in the cocaine-paired context (Tukey test, p<0.001), but did not alter responding in the extinction context. As a result, following BM pretreatment, there were no differences between responding in the cocaine-paired context and the extinction context. The effect of BM pretreatment on active lever responding in the cocaine-paired context was independent of the particular hemisphere into which BM was administered for either the BLA (t(18)=0.66, p=0.52) or the OFC (t(18)=0.29, p=0.78).
Inhibition of intrahemispheric or interhemispheric connections between the BLA and OFC similarly attenuate drug context-induced reinstatement of cocaine-seeking behavior. The panels depict non-reinforced active and inactive lever responses (mean/1 h+SEM) during testing in the extinction context (EXT context) and the previously cocaine-paired context (COC context). Immediately before testing, vehicle (VEH) or baclofen plus muscimol (BM) was infused unilaterally into the BLA plus the contralateral OFC (a, d) or the ipsilateral OFC (b, e), or unilaterally into the OFC alone (c, f). Asterisks represent significant difference relative to responding in the extinction context (a, b, d, e: ANOVA context simple main effect, p<0.05; c: ANOVA context main effect, p<0.05). Daggers represent significant difference relative to VEH pretreatment (a, b, d, e: ANOVA treatment simple main effect, p<0.05).
After VEH pretreatment, the contralateral and ipsilateral BLA/OFC-cannulated groups exhibited a slight increase in the inactive lever responding on exposure to the previously cocaine-paired context relative to responding in the extinction context (Figures 3d and e; context main effect, F(1,18)=8.49, p=0.009). BM pretreatment impaired inactive lever responding relative to VEH pretreatment in a context-specific manner after administration into either the contralateral BLA/OFC or the ipsilateral BLA/OFC (treatment X context interaction, F(1,18)=10.08, p=0.005; treatment main effect, F(1,18)=10.83, p=0.004; only statistically significant effects are reported). Specifically, independent of surgery condition, BM pretreatment attenuated inactive lever responding relative to VEH pretreatment in the cocaine-paired context (Tukey test, p<0.05) but not in the extinction context. The effect of BM pretreatment on inactive lever responding in the cocaine-paired context was independent of the particular hemisphere into which BM was administered for either the BLA (t(18)=0.29, p=0.77) or the OFC (t(18)=0.64, p=0.53).
Effects of Unilateral OFC Functional Inactivation on Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior
During the reinstatement test sessions, the unilateral OFC-cannulated group exhibited an increase in non-reinforced active lever responding in the previously cocaine-paired context relative to responding in the extinction context following VEH pretreatment (Figure 3c; context main effect, F(1,8)=34.56, p=0.001). BM pretreatment administered unilaterally into the OFC did not alter active lever responding relative to VEH pretreatment in either context (treatment X context interaction, F(1,8)=0.07, p=0.80; treatment main effect, F(1,8)=0.01, p=0.91). Exposure to the cocaine-paired context did not alter responding on the inactive lever relative to responding in the extinction context (Figure 3f; context, F(1,8)=2.07, p=0.19), and BM pretreatment administered unilaterally into the OFC did not alter inactive lever responding relative to VEH pretreatment in either context (treatment X context interaction, F(1,8)=1.43, p=0.266; treatment main effect, F(1,8)=2.49, p=0.15).
Locomotor Activity
BM pretreatment failed to alter locomotor activity relative to VEH pretreatment in the contralateral BLA/OFC-cannulated, ipsilateral BLA/OFC-cannulated, and unilateral OFC-cannulated groups (Figures 4a–c). In all groups, the number of photobeam breaks decreased at a similar rate over the three 20-min intervals of the locomotor test session as the groups habituated to the novel context (all time main effects, all Fs ⩾45.77, p=0.0001; interval 1> intervals 2–3; Tukey test, p<0.01). In addition, BM pretreatment did not alter the number of photobeam breaks relative to VEH pretreatment (all treatment main effects and interactions, all Fs ⩽0.105, p⩾0.072).
Inhibition of intrahemispheric or interhemispheric connections between the BLA and OFC does not alter locomotor activity measured as the number of photobeam breaks (mean/1 h+SEM) triggered by the movement of subjects in a novel context during a 1-h locomotor activity test. Immediately before testing, VEH or BM was infused unilaterally into the BLA plus the contralateral OFC (a) or the ipsilateral OFC (b), or unilaterally into the OFC alone (c). Plus signs represent significant difference relative to all other time points (a–c: ANOVA time simple main effect, interval 1> intervals 2–3, p<0.05).
Food-Reinforced Instrumental Behavior
BM pretreatment failed to alter food-reinforced instrumental performance relative to VEH pretreatment in the contralateral or ipsilateral BLA/OFC-cannulated groups (Figures 5a and b). Independent of surgery condition and treatment, all groups exhibited more active lever responding than inactive lever responding (lever main effect, F(1,13)=110.33, p=0.0001). Furthermore, BM pretreatment administered unilaterally into the BLA plus the contralateral or ipsilateral OFC did not alter food-reinforced responding alone or as a function of surgery condition or lever (treatment main effect and all treatment interactions, all Fs <0.46, p>0.51).
Inhibition of intrahemispheric or interhemispheric connections between the BLA and OFC fails to alter food-reinforced instrumental responding. The panels depict active and inactive lever responses (mean/1 h+SEM) during testing in the food self-administration context. Immediately before testing, BM or VEH was infused unilaterally into the BLA plus the contralateral OFC (a) or the ipsilateral OFC (b). BM treatment did not alter active or inactive lever responding relative to VEH treatment. Daggers represent significant difference relative to responding on the inactive lever (a, b, ANOVA lever main effect, p<0.05).
DISCUSSION
This study explored putative functionally significant interactions between the BLA and OFC in drug context-induced cocaine-seeking behavior. To this end, the effects of unilateral functional inactivation of the BLA plus the contralateral or ipsilateral OFC were assessed on the expression of cocaine seeking elicited by re-exposure to a drug-paired environmental context. Contralateral or ipsilateral administration of BM into the BLA plus OFC produced a profound attenuation of the reinstatement of drug context-induced cocaine seeking (Figures 3a and b). Although some drug context-induced cocaine-seeking behavior was also recorded on the inactive lever (Figures 3d and e), this phenomenon is often observed when behavioral conditioning occurs in the absence of an explicit cocaine-paired conditioned stimulus (Fuchs et al, 2007; Fuchs et al, 2009; Xie et al, 2010). Furthermore, this alternate form of cocaine-seeking behavior was also impaired by BM treatment. Importantly, BM-induced decreases in drug context-induced cocaine seeking were unlikely to reflect nonspecific deficits in instrumental motor performance, given that functional inactivation of the contralateral or ipsilateral BLA plus OFC did not alter active lever responding in the extinction context (Figures 3a and b), general motor activity in a novel context (Figures 4a–c), or food-reinforced instrumental behavior (Figures 5a and b). In addition, previous findings from our laboratory and from other investigators have demonstrated that bilateral functional inactivation of the OFC and BLA fails to alter cocaine-primed reinstatement of cocaine seeking (Lasseter and Fuchs, unpublished observation; Grimm and See, 2000). Overall, these findings indicate that neural activity in both the BLA and OFC is necessary for using the memory or motivational significance of cocaine-associated environmental stimuli to guide goal-directed behavior. Such results are consistent with previous research indicating the BLA and OFC are integral parts of the mesocorticolimbic neural circuitry known to direct cue and context-induced cocaine-seeking behavior in animal models of drug relapse (Grimm and See 2000; Neisewander et al, 2000; See et al, 2001; Kantak et al, 2002; McLaughlin and See 2003; Fuchs et al, 2004; Fuchs et al, 2005; Marinelli et al, 2007; Crombag et al, 2008; Fuchs et al, 2008a; Hamlin et al, 2008; Zavala et al, 2008; Lasseter et al, 2009; Mashhoon et al. 2010; Marinelli et al, 2010). Moreover, this study significantly extends this line of research by suggesting that the BLA and OFC co-regulate drug context-induced cocaine seeking via sequential information processing or by providing necessary input to a common downstream target within a neural circuit.
When interpreting the finding that ipsilateral and contralateral BLA/OFC neural inactivation produced similar impairment in cocaine seeking, it is important to note that rats exhibited robust drug context-induced cocaine-seeking behavior following unilateral functional inactivation of the OFC (Figure 3c) or BLA (Fuchs et al, 2007). Our findings are consistent with previous studies demonstrating that unilateral BLA or OFC manipulations are insufficient to disrupt the acquisition of reversal learning (Saddoris et al, 2005) or the expression of conditioned appetitive behaviors, including drug context-induced cocaine seeking and sucrose-conditioned place preference (Everitt et al, 1991; Fuchs et al, 2007), even though these manipulations are capable of disrupting some forms of conditioned learning and reward processing (LaBar and LeDoux 1996; Izquierdo and Murray 2004; Markham et al, 2010). Thus, one possible interpretation of these findings is that the ipsilateral and contralateral BLA/OFC manipulations crossed the threshold of neural inactivation sufficient to disrupt drug context-induced cocaine seeking independent of functional connectivity between the BLA and OFC. However, given that unilateral functional inactivation of either the BLA or OFC failed to alter the motivational significance of the cocaine-paired environmental context, it is unlikely that additive effects of these manipulations accounts for the robust effects of both the contralateral and ipsilateral BLA/OFC inactivation observed in this study, even if we are dealing with a nonlinear system.
A more likely possibility is that functionally significant interactions between the BLA and OFC may be necessary for the control of drug context-induced cocaine-seeking behavior. Given that the magnitude of impairment in context-induced cocaine seeking was similar following ipsilateral and contralateral neural inactivation of the BLA/OFC, the ability of the cocaine-paired context to elicit cocaine seeking may rely equally on the functional integrity of intrahemispheric and interhemispheric connections between the BLA and OFC, which were bilaterally disrupted by the contralateral and ipsilateral BM manipulations, respectively. This explanation is supported by considerable anatomical evidence indicating that the BLA and OFC share dense reciprocal intra- and interhemispheric projections that are topographically organized (Krettek and Price 1977; McDonald 1991; Barbas and Blatt 1995; Carmichael and Price 1995; Ghashghaei and Barbas 2002). Additional connections between the BLA and OFC are relayed through the mediodorsal thalamus (MDT), providing an anatomical substrate for extensive functional interactions between the BLA and OFC (Porrino et al, 1981; Barbas and Pandya 1984; Demeter et al, 1990; Cavada et al, 2000; Miyashita et al, 2007). Interestingly, amygdalocortical and amygdalothalamic pathways to the OFC involve distinct subpopulations of neurons within the BLA and OFC, indicating that these parallel pathways may convey functionally distinct information between the BLA and the OFC (Porrino et al, 1981; McDonald 1991; Miyashita et al, 2007).
The explanation that communication between the BLA and OFC subserves drug-seeking behaviors is further supported by evidence that functional interdependence exists between these brain regions in the regulation of other goal-directed behaviors. Indicating the importance of intrahemispheric communication between the BLA and OFC, previous studies have demonstrated that contralateral, although not ipsilateral, BLA/OFC neural inactivation disrupts performance on an odor reward-reversal task (Churchwell et al, 2009), whereas contralateral BLA/OFC lesions disrupt affective processing as evidenced by attenuated reinforcer devaluation effects and impaired object reversal learning (Baxter et al, 2000; Izquierdo and Murray, 2004). Furthermore, electrophysiological studies have confirmed that intrahemispheric interactions between the BLA and OFC promote behavioral flexibility on an odor discrimination task, although putative interhemispheric interactions were not similarly explored (Saddoris et al, 2005). Interestingly, however, contralateral BLA plus OFC lesions only transiently disrupt performance on a reinforcer devaluation task in contrast to the enduring behavioral deficits produced by bilateral BLA or OFC lesions (Izquierdo and Murray, 2004; Izquierdo et al, 2004; Izquierdo and Murray 2007, 2010). Thus, at least in the reinforcer devaluation task, recovery of function may occur following the permanent disruption of intrahemispheric interactions through the strengthening of interhemispheric functional connectivity between the intact brain regions, which underscores the importance of these pathways.
In conclusion, results from this study provide important evidence that interaction between the BLA and OFC is necessary for the expression of drug context-induced motivation for cocaine. This form of cocaine-seeking behavior may depend on intrahemispheric and interhemispheric information processing by the BLA and OFC via direct reciprocal anatomical projections or via the convergence of requisite information from both of these brain regions onto a third brain region within the circuitry. As noted above, one particular region with which the BLA and OFC may interact to direct cocaine-seeking behavior is the MDT given that the MDT makes similar contributions to conditioned behaviors as the BLA and OFC (Aggleton and Mishkin 1983; Gaffan and Murray 1990; Corbit et al, 2003). Moreover, a crossed-disconnection procedure indicated that the BLA and OFC interact with the MDT in the regulation of reward-based decision making (Izquierdo and Murray, 2010). In addition, the BLA and OFC send afferents to several elements of the neural circuitry that mediates context-induced drug-seeking behavior, including the nucleus accumbens (NAc), dorsal hippocampus (DH), dorsal medial prefrontal cortex (dmPFC), and ventral tegmental area (VTA) (Christie et al, 1987; McDonald 1991; Ray and Price 1992; Brog et al, 1993; Haber et al, 1995; O'Donnell and Grace 1995; Groenewegen et al, 1996; Pikkarainen et al, 1999; Bossert et al, 2004; Fuchs et al, 2005; Bossert et al, 2007). In particular, our laboratory has demonstrated that interactions between the BLA and DH as well as between the BLA and dmPFC, are necessary for drug context-induced cocaine-seeking behavior (Fuchs et al, 2007). Furthermore, interactions between the BLA and NAc may also be necessary for this behavior given that communication between these brain regions promotes responding for sucrose- and cocaine-paired conditioned stimuli (Setlow et al, 2002; Ambroggi et al, 2008; Di Ciano, 2008) and is critical for the expression of sucrose-conditioned place preference (Everitt et al, 1991). Finally, dopamine input from the VTA may regulate BLA–OFC interactions in context-induced cocaine-seeking behavior given that dopamine D1 receptor antagonism in either the BLA or OFC is sufficient to impair the acquisition and expression of cue-induced cocaine seeking and decreases the break point under a progressive ratio schedule for food reinforcement, respectively (See et al, 2001; Cetin et al, 2004; Berglind et al, 2006). Because the BLA and OFC exert important control over the motivational aspects of drug-paired environmental stimuli, further explication of the larger neural circuitry within which they interact to direct drug-seeking behavior may provide insight into the prevention of environmentally-induced drug relapse.
References
Aggleton JP, Mishkin M (1983). Memory impairments following restricted medial thalamic lesions in monkeys. Exp Brain Res 52: 199–209.
Alleweireldt AT, Hobbs RJ, Taylor AR, Neisewander JL (2006). Effects of SCH-23390 infused into the amygdala or adjacent cortex and basal ganglia on cocaine seeking and self-administration in rats. Neuropsychopharmacology 31: 363–374.
Ambroggi F, Ishikawa A, Fields HL, Nicola SM (2008). Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron 59: 648–661.
Barbas H, Pandya DN (1984). Topography of commissural fibers of the prefrontal cortex in the rhesus monkey. Exp Brain Res 55: 187–191.
Barbas H, Blatt GJ (1995). Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5: 511–533.
Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA (2000). Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci 20: 4311–4319.
Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE (2006). Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience 137: 699–706.
Bossert JM, Liu SY, Lu L, Shaham Y (2004). A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci 24: 10726–10730.
Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y (2007). Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27: 12655–12663.
Brog JS, Salyapongse A, Deutch AY, Zahm DS (1993). The patterns of afferent innervation of the core and shell in the ‘accumbens’ part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol 338: 255–278.
Carmichael ST, Price JL (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363: 615–641.
Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F (2000). The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex 10: 220–242.
Cetin T, Freudenberg F, Fuchtemeier M, Koch M (2004). Dopamine in the orbitofrontal cortex regulates operant responding under a progressive ratio of reinforcement in rats. Neurosci Lett 370: 114–117.
Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O'Brien CP (1993). Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137: 73–95.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18.
Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM (1987). Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience 22: 425–439.
Churchwell JC, Morris AM, Heurtelou NM, Kesner RP (2009). Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci 123: 1185–1196.
Corbit LH, Muir JL, Balleine BW (2003). Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. Eur J Neurosci 18: 1286–1294.
Crombag HS, Grimm JW, Shaham Y (2002). Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology 27: 1006–1015.
Crombag HS, Bossert JM, Koya E, Shaham Y (2008). Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci 363: 3233–3243.
Demeter S, Rosene DL, Van Hoesen GW (1990). Fields of origin and pathways of the interhemispheric commissures in the temporal lobe of macaques. J Comp Neurol 302: 29–53.
Di Ciano P (2008). Drug seeking under a second-order schedule of reinforcement depends on dopamine D3 receptors in the basolateral amygdala. Behav Neurosci 122: 129–139.
Ehrman RN, Robbins SJ, Childress AR, O'Brien CP (1992). Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology 107: 523–529.
Everitt BJ, Morris KA, O'Brien A, Robbins TW (1991). The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience 42: 1–18.
Foltin RW, Haney M (2000). Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 149: 24–33.
Fuchs RA, Evans KA, Parker MP, See RE (2004). Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci 24: 6600–6610.
Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH et al. (2005). The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30: 296–309.
Fuchs RA, Eaddy JL, Su ZI, Bell GH (2007). Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci 26: 487–498.
Fuchs RA, Lasseter HC, Ramirez DR, Xie X (2008a). Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models 5: 251–258.
Fuchs RA, Ramirez DR, Bell GH (2008b). Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 200: 545–556.
Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su ZI (2009). Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci 30: 889–900.
Gaffan D, Murray EA (1990). Amygdalar interaction with the mediodorsal nucleus of the thalamus and the ventromedial prefrontal cortex in stimulus-reward associative learning in the monkey. J Neurosci 10: 3479–3493.
Ghashghaei HT, Barbas H (2002). Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115: 1261–1279.
Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C et al. (1996). Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93: 12040–12045.
Grimm JW, See RE (2000). Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology 22: 473–479.
Groenewegen HJ, Wright CI, Beijer AV (1996). The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res 107: 485–511.
Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E (1995). The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 15: 4851–4867.
Hamlin AS, Clemens KJ, McNally GP (2008). Renewal of extinguished cocaine-seeking. Neuroscience 151: 659–670.
Hearing MC, Miller SW, See RE, McGinty JF (2008a). Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology (Berl) 198: 77–91.
Hearing MC, See RE, McGinty JF (2008b). Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct Funct 213: 215–227.
Izquierdo A, Murray EA (2004). Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J Neurophysiol 91: 2023–2039.
Izquierdo A, Suda RK, Murray EA (2004). Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci 24: 7540–7548.
Izquierdo A, Murray EA (2007). Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning. J Neurosci 27: 1054–1062.
Izquierdo A, Murray EA (2010). Functional interaction of medial mediodorsal thalamic nucleus but not nucleus accumbens with amygdala and orbital prefrontal cortex is essential for adaptive response selection after reinforcer devaluation. J Neurosci 30: 661–669.
Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB (2002). Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22: 1126–1136.
Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F et al. (2001). Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58: 334–341.
Krettek JE, Price JL (1977). Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol 172: 687–722.
LaBar KS, LeDoux JE (1996). Partial disruption of fear conditioning in rats with unilateral amygdala damage: correspondence with unilateral temporal lobectomy in humans. Behav Neurosci 110: 991–997.
Lasseter HC, Ramirez DR, Xie X, Fuchs RA (2009). Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci 30: 1370–1381.
London ED, Bonson KR, Ernst M, Grant S (1999). Brain imaging studies of cocaine abuse: implications for medication development. Crit Rev Neurobiol 13: 227–242.
Marinelli PW, Funk D, Juzytsch W, Li Z, Le AD (2007). Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci 26: 2815–2823.
Marinelli PW, Funk D, Juzytsch W, Le AD (2010). Opioid receptors in the basolateral amygdala but not dorsal hippocampus mediate context-induced alcohol seeking. Behav Brain Res 211: 58–63.
Markham CM, Taylor SL, Huhman KL (2010). Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem 17: 109–116.
Martin JH, Ghez C (1999). Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods 86: 145–159.
Mashhoon Y, Wells AM, Kantak KM (2010). Interaction of the rostral basolateral amygdala and prelimbic prefrontal cortex in regulating reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav 96: 347–353.
McDonald AJ (1991). Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44: 1–14.
McLaughlin J, See RE (2003). Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 168: 57–65.
Miyashita T, Ichinohe N, Rockland KS (2007). Differential modes of termination of amygdalothalamic and amygdalocortical projections in the monkey. J Comp Neurol 502: 309–324.
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF (2000). Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20: 798–805.
O'Donnell P, Grace AA (1995). Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15: 3622–3639.
Paxinos G, Watson C 1997. The Rat Brain Stereotaxic Coordinates. Academic Press: New York.
Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A (1999). Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol 403: 229–260.
Porrino LJ, Crane AM, Goldman-Rakic PS (1981). Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. J Comp Neurol 198: 121–136.
Ray JP, Price JL (1992). The organization of the thalamocortical connections of the mediodorsal thalamic nucleus in the rat, related to the ventral forebrain-prefrontal cortex topography. J Comp Neurol 323: 167–197.
Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM (1990). Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict 25: 957–993.
Saddoris MP, Gallagher M, Schoenbaum G (2005). Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron 46: 321–331.
See RE, Kruzich PJ, Grimm JW (2001). Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 154: 301–310.
See RE, Fuchs RA, Ledford CC, McLaughlin J (2003). Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci 985: 294–307.
Setlow B, Gallagher M, Holland PC (2002). The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. Eur J Neurosci 15: 1841–1853.
Takahashi YK, Roesch MR, Stalnaker TA, Haney RZ, Calu DJ, Taylor AR et al. (2009). The orbitofrontal cortex and ventral tegmental area are necessary for learning from unexpected outcomes. Neuron 62: 269–280.
Xie X, Ramirez DR, Lasseter HC, Fuchs RA (2010). Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 208: 1–11.
Zavala AR, Osredkar T, Joyce JN, Neisewander JL (2008). Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse 62: 421–431.
Acknowledgements
We thank Kate Cowhey, Trey Newsome, John Tobben, Portia West, and Amy Zipursky for their excellent technical assistance and insightful comments on an earlier version of this manuscript. This work was supported by National Institute on Drug Abuse R01 DA017673, DA025646, and DA025617.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lasseter, H., Wells, A., Xie, X. et al. Interaction of the Basolateral Amygdala and Orbitofrontal Cortex is Critical for Drug Context-Induced Reinstatement of Cocaine-Seeking Behavior in Rats. Neuropsychopharmacol 36, 711–720 (2011). https://doi.org/10.1038/npp.2010.209
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2010.209
Keywords
This article is cited by
-
Evaluation of the 5-HT2C receptor drugs RO 60-0175, WAY 161503 and mirtazepine in a preclinical model of comorbidity of depression and cocaine addiction
Pharmacological Reports (2023)
-
Contribution of the prefrontal cortex and basolateral amygdala to behavioral decision-making under reward/punishment conflict
Psychopharmacology (2020)
-
Role of adolescent-formed, context-drug-associations on reinstatement of drug-seeking behavior in rats
Psychopharmacology (2020)
-
Common neurocircuitry mediating drug and fear relapse in preclinical models
Psychopharmacology (2019)
-
Role of a Lateral Orbital Frontal Cortex-Basolateral Amygdala Circuit in Cue-Induced Cocaine-Seeking Behavior
Neuropsychopharmacology (2017)