Abstract
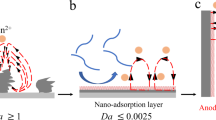
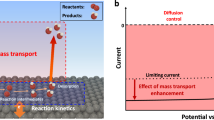
A concentration cell1,2,3 is composed of two equivalent half-cells made of the same material but differing in the concentration of reactants. As these concentrations equilibrate, the increase in entropy is converted into a flow of electricity with the voltage output determined by the Nernst equation and proportional to the logarithm of the concentration ratios. However, as diffusion constantly strives to erase all concentration gradients4,5,6, concentration cells produce only moderate voltages (typically tens of millivolts at room temperature7,8) over relatively short times9 and, consequently, such devices have not been regarded as promising for energy storage10,11,12. Here, we report a concentration cell that produces significantly higher voltages (∼0.5 V) for over 100 h. The key to our design is that the citric acid molecules involved in the electrode reactions are tethered onto magnetic nanoparticles, and a sharp gradient (107–1011 anode/cathode concentration ratio) is maintained at one of the electrodes by a permanent magnet external to the cell. Our cell does not result in corrosion of the electrodes, produces no harmful by-products, and can be regenerated by recoating used nanoparticles with fresh citric acid. We show that a series of such centimetre-sized cells produces enough electricity to power small electronic devices (timers and calculators) for several tens of hours. Our results illustrate how redox-active molecules that are, in themselves, non-magnetic can be effectively concentrated by magnetic fields to produce electrical energy13,14,15.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bard, A. J. & Faulkner, L. R. Electrochemical Methods: Fundamentals and Applications Vol. 2 (Wiley, 1980).
Bockris, J. O. M. & Reddy, A. K. Modern Electrochemistry: An Introduction to an Interdisciplinary Area Vol. 2 (Springer, 1973).
Weinstein, J. N. & Leitz, F. B. Electric power from difference in salinity: the dialytic battery. Science 191, 557–559 (1976).
Grzybowski, B. Chemistry in Motion: Reaction–Diffusion Systems for Micro- and Nanotechnology (Wiley, 2009).
Grzybowski, B. A., Bishop, K. J., Campbell, C. J., Fialkowski, M. & Smoukov, S. K. Micro-and nanotechnology via reaction–diffusion. Soft Matter 1, 114–128 (2005).
Soh, S., Byrska, M., Kandere-Grzybowska, K. & Grzybowski, B. A. Reaction–diffusion systems in intracellular molecular transport and control. Angew. Chem. Int. Ed. 49, 4170–4198 (2010).
Lagger, G., Jensen, H., Josserand, J. & Girault, H. H. Hydro-voltaic cells: Part I. Concentration cells. J. Electroanal. Chem. 545, 1–6 (2003).
Banan Sadeghian, R., Pantchenko, O., Tate, D. & Shakouri, A. Miniaturized concentration cells for small-scale energy harvesting based on reverse electrodialysis. Appl. Phys. Lett. 99, 173702 (2011).
Xu, J. & Lavan, D. A. Designing artificial cells to harness the biological ion concentration gradient. Nature Nanotech. 3, 666–670 (2008).
Guo, W. et al. Energy harvesting with single-ion-selective nanopores: A concentration-gradient-driven nanofluidic power source. Adv. Funct. Mater. 20, 1339–1344 (2010).
Xu, J., Sigworth, F. J. & LaVan, D. A. Synthetic protocells to mimic and test cell function. Adv. Mater. 22, 120–127 (2010).
Komhyr, W., Barnes, R., Brothers, G., Lathrop, J. & Opperman, D. Electrochemical concentration cell ozonesonde performance evaluation during STOIC 1989. J. Geophys. Res. D 100, 9231–9244 (1995).
Sakai, H. et al. A high-power glucose/oxygen biofuel cell operating under quiescent conditions. Energy Environ. Sci. 2, 133–138 (2009).
Gao, F., Viry, L., Maugey, M., Poulin, P. & Mano, N. Engineering hybrid nanotube wires for high-power biofuel cells. Nature Commun. 1, 2 (2010).
O'Regan, B. & Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991).
Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 17, 1247–1248 (1981).
Sahoo, Y. et al. Aqueous ferrofluid of magnetite nanoparticles: fluorescence labeling and magnetophoretic control. J. Phys. Chem. B 109, 3879–3885 (2005).
Sze, S. M. & Ng, K. K. Physics of Semiconductor Devices (Wiley, 2006).
Kalsin, A. M. et al. Electrostatic self-assembly of binary nanoparticle crystals with a diamond-like lattice. Science 312, 420–424 (2006).
Jones, D. A. Principles and Prevention of Corrosion (Prentice Hall, 1996).
Mekki, A., Holland, D., McConville, C. F. & Salim, M. An XPS study of iron sodium silicate glass surfaces. J. Non-Cryst. Solids 208, 267–276 (1996).
Yamashita, T. & Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 254, 2441–2449 (2008).
Bard, A. J., Parsons, R. & Jordan, J. Standard Potentials in Aqueous Solutions Vol. 6 (CRC Press, 1985).
Turkevich, J., Stevenson, P. C. & Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 11, 55–75 (1951).
Lee, P. C. & Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 86, 3391–3395 (1982).
John, M. L. Methods in Enzymology Citric acid cycle Vol. 13 (Academic, 1969).
Kuyper, A. C. The oxidation of citric acid. J. Am. Chem. Soc. 55, 1722–1727 (1933).
Munro, C., Smith, W., Garner, M., Clarkson, J. & White, P. Characterization of the surface of a citrate-reduced colloid optimized for use as a substrate for surface-enhanced resonance Raman scattering. Langmuir 11, 3712–3720 (1995).
Tabrizi, A., Ayhan, F. & Ayhan, H. Gold nanoparticle synthesis and characterisation. J. Biol. Chem. 37, 217–226 (2009).
Müller, H. M. & Frosch, S. Oxalate accumulation from citrate by Aspergillus niger. II. Involvement of the tricarboxylic acid cycle. Arch. Microbiol. 104, 159–162 (1975).
Van Rijssel, J., Kuipers, B. W. M. & Erné, B. H. Non-regularized inversion method from light scattering applied to ferrofluid magnetization curves for magnetic size distribution analysis. J. Magn. Magn. Mater. 353, 110–115 (2014).
Rightmire, R., Rowland, R., Boos, D. & Beals, D. Ethyl alcohol oxidation at platinum electrodes. J. Electrochem. Soc. 111, 242–247 (1964).
Park, S. M., Chen, N. C. & Doddapaneni, N. Electrochemical oxidation of ethanol in aqueous carbonate solutions. J. Electrochem. Soc. 142, 40–45 (1995).
Acknowledgements
This work was supported by the Non-Equilibrium Energy Research Center (NERC), which is an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences (award no. DE-SC0000989). J.V.I.T. was supported by a Walter Ahlström Foundation postdoctoral grant. The authors thank B. Kowalczyk and P. Pillai for discussions.
Author information
Authors and Affiliations
Contributions
Y.Y. carried out most experiments and performed data analysis. J.V.I.T. synthesized and characterized the Fe3O4 nanoparticles and analysed the data. Y.Y., J.V.I.T. and B.A.G. wrote the manuscript. Y.Y. and B.A.G. conceived the project. B.A.G. supervised the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Yan, Y., Timonen, J. & Grzybowski, B. A long-lasting concentration cell based on a magnetic electrolyte. Nature Nanotech 9, 901–906 (2014). https://doi.org/10.1038/nnano.2014.198
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2014.198
This article is cited by
-
A nanogenerator based on metal nanoparticles and magnetic ionic gradients
NPG Asia Materials (2023)
-
Local enhancement of concentration gradient through the hydrogel-functionalized anodic aluminum oxide membranes for osmotic power generation
Macromolecular Research (2023)
-
Water-evaporation-induced electricity with nanostructured carbon materials
Nature Nanotechnology (2017)
-
Nanometer-size hard magnetic ferrite exhibiting high optical-transparency and nonlinear optical-magnetoelectric effect
Scientific Reports (2015)