Abstract
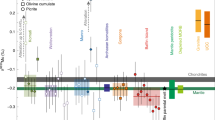
The global boron geochemical cycle is closely linked to recycling of geologic material via subduction processes that have occurred over billions of years of Earth’s history. The origin of carbonatites, unique melts derived from carbon-rich and carbonate-rich regions of the upper mantle, has been linked to a variety of mantle-related processes, including subduction and plume–lithosphere interaction. Here we present boron isotope (δ11B) compositions for carbonatites from locations worldwide that span a wide range of emplacement ages (between ∼40 and ∼2,600 Ma). Hence, they provide insight into the temporal evolution of their mantle sources for ∼2.6 billion years of Earth’s history. Boron isotope values are highly variable and range between −8.6‰ and +5.5‰, with all of the young (<300 Ma) carbonatites characterized by more positive δ11B values (>−4.0‰), whereas most of the older carbonatite samples record lower B isotope values. Given the δ11B value for asthenospheric mantle of −7 ± 1‰, the B isotope compositions for young carbonatites require the involvement of an enriched (crustal) component. Recycled crustal components may be sampled by carbonatite melts associated with mantle plume activity coincident with major tectonic events, and linked to past episodes of significant subduction associated with supercontinent formation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chaussidon, M. & Jambon, A. Boron content and isotopic composition of oceanic basalts: geochemical and cosmochemical implications. Earth Planet. Sci. Lett. 259, 541–556 (1994).
Spivack, A. J. & Edmond, J. M. Boron isotope exchange between seawater and the oceanic crust. Geochim. Cosmochim. Acta 51, 1033–1043 (1987).
Palmer, M. R. & Swihart, G. H. Boron isotope geochemistry: an overview. Rev. Mineral. 33, 709–744 (1996).
Konrad-Schmolke, M. & Halama, R. Combined thermodynamic-geochemical modeling in metamorphic geology: boron as tracer of fluid-rock interaction. Lithos 208–209, 393–414 (2014).
Wunder, B., Meixner, A., Romer, R. L., Wirth, R. & Heinrich, W. The geochemical cycle of boron: constraints from boron isotope partitioning experiments between mica and fluid. Lithos 84, 206–216 (2005).
Woolley, A. R. & Kjarsgaard, B. A. Carbonatite Occurrences of the World: Map and Database (Geological Survey of Canada, 2008).
Luth, R. W. Diamonds, eclogites, and the oxidation-state of the Earth’s mantle. Science 261, 66–68 (1993).
Dasgupta, R. & Hirschmann, M. M. Melting in the Earth’s deep upper mantle caused by carbon dioxide. Nature 440, 659–662 (2006).
Yaxley, G. M., Green, D. H. & Kamenetsky, V. Carbonatite metasomatism in the southeastern Australian lithosphere. J. Petrol. 39, 1917–1930 (1998).
Rukhlov, A. S. & Bell, K. Geochronology of carbonatites from the Canadian and Baltic Shields, and the Canadian Cordillera: clues to mantle evolution. Mineral. Petrol. 98, 11–54 (2010).
Bell, K., Blenkinsop, J., Cole, T. J. S. & Menagh, D. P. Evidence from Sr isotopes for long-lived heterogeneities in the upper mantle. Nature 298, 251–253 (1982).
Halama, R., McDonough, W. F., Rudnick, R. L. & Bell, K. Tracking the lithium isotopic evolution of the mantle using carbonatites. Earth Planet. Sci. Lett. 265, 726–742 (2008).
Woolley, A. R. The Spatial and Temporal Distribution of Carbonatites: Carbonatites: Genesis and EvolutionCh. 2 (Unwin Hyman, 1989).
Veizer, J., Bell, K. & Jansen, S. L. Temporal distribution of carbonatites. Geology 20, 1147–1149 (1992).
Bell, K. & Simonetti, A. Source of parental melts to carbonatites–critical isotopic constraints. Mineral. Petrol. 98, 77–89 (2010).
Bell, K. & Tilton, G. R. Probing the mantle: the story from carbonatites. Eos Trans. Am. Geophys. Union 83, 273–280 (2002).
Zindler, A. & Hart, S. R. Chemical dynamics. Annu. Rev. Earth Planet. Sci. 14, 493–571 (1986).
Weiss, Y., Class, C., Goldstein, S. L. & Hanyu, T. Key new pieces of the HIMU puzzle from olivines and diamond inclusions. Nature 537, 666–670 (2016).
Bizzarro, M., Simonetti, A., Stevenson, R. K. & David, J. Hf isotope evidence for a hidden mantle reservoir. Geology 30, 771–774 (2002).
Barker, D. S. Consequences of recycled carbon in carbonatites. Can. Mineral. 34, 373–387 (1996).
Hoernle, K., Tilton, G., Le Bas, M. J., Duggen, S. & Garbe-Schönberg, D. Geochemistry of oceanic carbonatites compared with continental carbonatites: mantle recycling of oceanic crustal carbonate. Contrib. Mineral. Petrol. 142, 520–542 (2002).
D’Orazio, M., Innocenti, F., Tonarini, S. & Doglioni, C. Carbonatites in a subduction system: the Pleistocene alvikites from Mt. Vulture (southern Italy). Lithos 98, 313–334 (2007).
Bell, K., Lavecchia, G. & Rosatelli, G. Cenozoic Italian magmatism—Isotope constraints for possible plume-related activity. J. South Am. Earth Sci. 41, 22–40 (2013).
Mitchell, R. H. Carbonatites and carbonatites and carbonatites. Can. Mineral. 43, 2049–2068 (2005).
Smart, K. A., Tappe, S., Stern, R. A., Webb, S. J. & Ashwal, L. D. Early Archaean tectonics and mantle redox recorded in Witwatersrand diamonds. Nat. Geosci. 9, 255–259 (2016).
Hulett, S. W. R. Boron Abundances and Isotope Systematics of Carbonatites from Worldwide Sources MS thesis, Univ. Notre Dame (2016).
Chaussidon, M. & Marty, B. Primitive boron isotope composition of the mantle. Science 269, 383–386 (1995).
Keller, J. & Hoefs, J. Stable Isotope Characteristics of Recent Natrocarbonatites from Oldoinyo Lengai: Carbonatite Volcanism IAVCEI Proceedings in Volcanology Vol. 4 (Springer, 1995).
Deines, P. Stable Isotope Variations in Carbonatites: Carbonatites: Genesis and EvolutionCh. 13 (Unwin Hyman, 1989).
Turner, S., Tonarini, S., Bindeman, I., Leeman, W. P. & Schaefer, B. F. Boron and oxygen isotope evidence for recycling of subducted components over the past 2.5 Gyr. Nature 447, 702–705 (2007).
Genske, F. S. et al. Lithium and boron isotope systematics in lavas from the Azores islands reveal crustal assimilation. Chem. Geol. 273, 27–36 (2014).
Jones, A. P., Genge, M. & Carmody, L. Carbonate melts and carbonatites. Rev. Mineral. Geochem. 75, 289–322 (2013).
Simonetti, A., Goldstein, S. L., Schmidberger, S. S. & Viladkar, S. G. Geochemical and Nd, Pb, and Sr isotope data from Deccan alkaline complexes—inferences for mantle sources and plume–lithosphere interaction. J. Petrol. 39, 1847–1864 (1998).
Meert, J. G. A synopsis of events related to the assembly of eastern Gondwana. Tectonophysics 362, 1–40 (2003).
Zhao, G., Sun, M., Wilde, S. A. & Li, S. Assembly, accretion and breakup of the Paleo-Mesoproterozoic Columbia Supercontinent: records in the North China Craton. Gondwana Res. 6, 417–434 (2003).
Shaw, A. M. et al. Long-term preservation of slab signatures in the mantle inferred from hydrogen isotopes. Nat. Geosci. 5, 224–228 (2012).
Hammouda, T. High-pressure melting of carbonated eclogite and experimental constraints on carbon recycling and storage in the mantle. Earth Planet. Sci. Lett. 214, 357–368 (2003).
Yaxley, G. M. & Brey, G. P. Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: implications for petrogenesis of carbonatites. Contrib. Mineral. Petrol. 146, 606–619 (2004).
Dasgupta, R., Hirschmann, M. M. & Withers, A. W. Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite. Earth Planet. Sci. Lett. 227, 73–85 (2004).
Dasgupta, R. Ingassing, storage, and outgassing of terrestrial carbon through geologic time. Rev. Mineral. Geochem. 75, 183–229 (2013).
Lay, T., Hernlund, J. & Buffett, B. A. Core-mantle boundary heat flow. Nat. Geosci. 1, 25–32 (2008).
Collins, W. J. Slab pull, mantle convection, and Pangaean assembly and dispersal. Earth Planet. Sci. Lett. 205, 225–237 (2003).
Stern, R. J. Evidence from ophiolites, blueschists, and ultrahigh-pressure metamorphic terranes that the modern episode of subduction tectonics began in Neoproterozoic time. Geology 33, 557–560 (2005).
van der Hilst, R. D., Widiyantoro, S. & Engdahl, E. R. Evidence for deep mantle circulation from global tomography. Nature 386, 578–584 (1997).
Ernst, R. E. & Bell, K. Large igneous provinces (LIPs) and carbonatites. Mineral. Petrol. 98, 55–76 (2010).
Maloof, A. C. et al. The earliest Cambrian record of animals and ocean geochemical change. Geol. Soc. Am. Bull. 122, 1731–1774 (2010).
Mirota, M. D. & Veizer, J. Geochemistry of Precambrian carbonates: VI. Aphebian Albanel Formations, Quebec, Canada. Geochim. Cosmochim. Acta 58, 1735–1745 (1994).
Bell, K. & Blenkinsop, J. Neodymium and Strontium Isotope Geochemistry of Carbonatites: Carbonatites: Genesis and EvolutionCh. 12 (Unwin Hyman, 1989).
Bell, K. & Tilton, G. R. Nd, Pb and Sr isotope compositions of East African carbonatites: evidence for mantle mixing and plume inhomogeneity. J. Petrol. 42, 1927–1945 (2001).
Zagnitko, V., Kryvdik, S. & Donskiy, M. Isotope geochemistry of carbonatites of Ukraine. Period. Mineral. 72, 153–159 (2003).
Chen, W. & Simonetti, A. Isotopic (Pb, Sr, Nd, C, O) evidence for plume-related sampling of an ancient, depleted mantle reservoir. Lithos 216–217, 81–92 (2015).
Foster, G. L. Seawater pH, pCO2 and [CO32−] variations in the Caribbean Sea over the last 130 kyr: a boron isotope and B/Ca study of planktic foraminifera. Earth Planet. Sci. Lett. 271, 254–266 (2008).
Lemarchand, D., Gaillardet, J., Göpel, C. & Manhes, G. An optimized procedure for boron separation and mass spectrometry analysis for river samples. Chem. Geol. 182, 323–334 (2002).
McCrea, J. M. On the isotopic chemistry of carbonates and a paleotemperature scale. J. Chem. Phys. 18, 849–857 (1950).
Coplen, T. B. et al. New guidelines for δ13C measurements. Anal. Chem. 78, 2439–2441 (2006).
Craig, H. Standard for reporting concentrations of deuterium and oxygen-18 in natural waters. Science 133, 1833–1834 (1961).
Acknowledgements
We thank K. Bell for donating most of the carbonatite samples investigated here. S. Hulett is appreciative of the assistance provided by D. Birdsell (Center for Environmental Science Technology (CEST), University of Notre Dame) in relation to the stable C and O isotope analyses and use of the micro-X-ray fluorescence instrument. I. Steele and B. Monaco are thanked for help with conducting EMP and LA-ICP-MS analyses. The work reported here was possible owing to financial support from the University of Notre Dame.
Author information
Authors and Affiliations
Contributions
A.S. and S.R.W.H. conceived the model and prepared the initial manuscript. S.R.W.H. conducted all of the analytical work reported here at the University of Notre Dame, and E.T.R. and N.G.H. supervised S.R.W.H. during a 1-week instructional visit at Stony Brook. E.T.R. conducted the ion exchange chemistry and B isotope analyses on four carbonatite samples at Stony Brook University. All authors contributed to preparation of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 3262 kb)
Rights and permissions
About this article
Cite this article
Hulett, S., Simonetti, A., Rasbury, E. et al. Recycling of subducted crustal components into carbonatite melts revealed by boron isotopes. Nature Geosci 9, 904–908 (2016). https://doi.org/10.1038/ngeo2831
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo2831
This article is cited by
-
H2O-induced sedimentary carbon migration from subducting slabs to the forearc mantle
Science China Earth Sciences (2022)
-
REE mineralization related to carbonatites and alkaline magmatism in the northern Tarim basin, NW China: implications for a possible Permian large igneous province
International Journal of Earth Sciences (2022)
-
Rapid recycling of subducted sedimentary carbon revealed by Afghanistan carbonatite volcano
Nature Geoscience (2021)
-
Carbonatites and Alkaline Igneous Rocks in Post-Collisional Settings: Storehouses of Rare Earth Elements
Journal of Earth Science (2021)
-
Ore-forming processes in the Khetri Copper Belt, western India: constraints from trace element chemistry of pyrite and C-O isotope composition of carbonates
Mineralium Deposita (2021)