Abstract
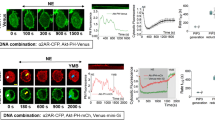
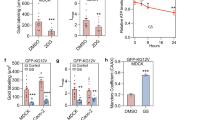
G protein–coupled receptors (GPCRs) transmit exogenous signals to the nucleus, promoting a myriad of biological responses via multiple signaling pathways in both healthy and cancerous cells. However, little is known about the response of cytosolic metabolic pathways to GPCR-mediated signaling. Here we applied fluorescent live-cell imaging and label-free dynamic mass redistribution assays to study whether purine metabolism is associated with GPCR signaling. Through a library screen of GPCR ligands in conjunction with live-cell imaging of a metabolic multienzyme complex for de novo purine biosynthesis, the purinosome, we demonstrated that the activation of endogenous Gαi-coupled receptors correlates with purinosome assembly and disassembly in native HeLa cells. Given the implications of GPCRs in mitogenic signaling and of the purinosome in controlling metabolic flux via de novo purine biosynthesis, we hypothesize that regulation of purinosome assembly and disassembly may be one of the downstream events of mitogenic GPCR signaling in human cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Neves, S.R., Ram, P.T. & Iyengar, R. G protein pathways. Science 296, 1636–1639 (2002).
Pierce, K.L., Premont, R.T. & Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3, 639–650 (2002).
Dorsam, R.T. & Gutkind, J.S. G-protein-coupled receptors and cancer. Nat. Rev. Cancer 7, 79–94 (2007).
Rozengurt, E. Mitogenic signaling pathways induced by G protein-coupled receptors. J. Cell. Physiol. 213, 589–602 (2007).
Rajagopal, S., Rajagopal, K. & Lefkowitz, R.J. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 9, 373–386 (2010).
Kroemer, G. & Pouyssegur, J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell 13, 472–482 (2008).
Kaelin, W.G. Jr. & Thompson, C.B. Q&A: Cancer: clues from cell metabolism. Nature 465, 562–564 (2010).
Denkert, C. et al. Metabolite profiling of human colon carcinoma—deregulation of TCA cycle and amino acid turnover. Mol. Cancer 7, 72 (2008).
Christopherson, R.I., Lyons, S.D. & Wilson, P.K. Inhibitors of de novo nucleotide biosynthesis as drugs. Acc. Chem. Res. 35, 961–971 (2002).
An, S., Kumar, R., Sheets, E.D. & Benkovic, S.J. Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 320, 103–106 (2008).
An, S., Kyoung, M., Allen, J.J., Shokat, K.M. & Benkovic, S.J. Dynamic regulation of a metabolic multi-enzyme complex by protein kinase CK2. J. Biol. Chem. 285, 11093–11099 (2010).
An, S., Deng, Y., Tomsho, J.W., Kyoung, M. & Benkovic, S.J. Microtubule-assisted mechanism for functional metabolic macromolecular complex formation. Proc. Natl. Acad. Sci. USA 107, 12872–12876 (2010).
Fang, Y., Ferrie, A.M., Fontaine, N.H. & Yuen, P.K. Characteristics of dynamic mass redistribution of epidermal growth factor receptor signaling in living cells measured with label-free optical biosensors. Anal. Chem. 77, 5720–5725 (2005).
Fang, Y., Li, G. & Ferrie, A.M. Non-invasive optical biosensor for assaying endogenous G protein-coupled receptors in adherent cells. J. Pharmacol. Toxicol. Methods 55, 314–322 (2007).
Schröder, R. et al. Deconvolution of complex G protein–coupled receptor signaling in live cells using dynamic mass redistribution measurements. Nat. Biotechnol. 28, 943–949 (2010).
Fang, Y. Label-free receptor assays. Drug Discov. Today Technol. 7, e5–e11 (2011).
Duncan, J.S. et al. An unbiased evaluation of CK2 inhibitors by chemoproteomics: characterization of inhibitor effects on CK2 and identification of novel inhibitor targets. Mol. Cell. Proteomics 7, 1077–1088 (2008).
Pagano, M.A. et al. The selectivity of inhibitors of protein kinase CK2: an update. Biochem. J. 415, 353–365 (2008).
Bylund, D.B. et al. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 46, 121–136 (1994).
Morris, A.J. & Malbon, C.C. Physiological regulation of G protein-linked signaling. Physiol. Rev. 79, 1373–1430 (1999).
Baker, J.G. The selectivity of beta-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br. J. Pharmacol. 144, 317–322 (2005).
Barbieri, J.T. & Cortina, G. ADP-ribosyltransferase mutations in the catalytic S-1 subunit of pertussis toxin. Infect. Immun. 56, 1934–1941 (1988).
Gill, D.M. & Meren, R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc. Natl. Acad. Sci. USA 75, 3050–3054 (1978).
Hanyaloglu, A.C. et al. Casein kinase II sites in the intracellular C-terminal domain of the thyrotropin-releasing hormone receptor and chimeric gonadotropin-releasing hormone receptors contribute to beta-arrestin-dependent internalization. J. Biol. Chem. 276, 18066–18074 (2001).
Musnier, A., Blanchot, B., Reiter, E. & Crepieux, P. GPCR signalling to the translation machinery. Cell. Signal. 22, 707–716 (2010).
Rebholz, H. et al. CK2 negatively regulates Gαs signaling. Proc. Natl. Acad. Sci. USA 106, 14096–14101 (2009).
Torrecilla, I. et al. Phosphorylation and regulation of a G protein-coupled receptor by protein kinase CK2. J. Cell Biol. 177, 127–137 (2007).
Gao, Y. & Wang, H.Y. Casein kinase 2 is activated and essential for Wnt/β-catenin signaling. J. Biol. Chem. 281, 18394–18400 (2006).
Acknowledgements
This work was funded by US National Institutes of Health grant GM24129 (S.J.B.).
Author information
Authors and Affiliations
Contributions
F.V. and S.A. contributed equally to the study. F.V. performed DMR assays and analyzed DMR data. S.A. performed fluorescent live-cell imaging and initial DMR assays, and analyzed data. A.M.F. prepared the compound library and performed initial DMR assays. H.S. performed quantitative real-time PCR. M.K. designed and modified a fluorescent microscope for image collection. H.D. carried out DMR assays related to part of Figure 3b, part of supplementary DMR assays. Y.F. and S.J.B. conceived and designed the study and analyzed data. S.A., Y.F. and S.J.B. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
F. Verrier, A.M. Ferrie, H. Sun, H. Deng and Y. Fang are employees and shareholders of Corning, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Results and Supplementary Methods (PDF 632 kb)
Rights and permissions
About this article
Cite this article
Verrier, F., An, S., Ferrie, A. et al. GPCRs regulate the assembly of a multienzyme complex for purine biosynthesis. Nat Chem Biol 7, 909–915 (2011). https://doi.org/10.1038/nchembio.690
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.690
This article is cited by
-
Human primary endothelial label-free biochip assay reveals unpredicted functions of plasma serine proteases
Scientific Reports (2020)
-
The role of dynamic enzyme assemblies and substrate channelling in metabolic regulation
Nature Communications (2018)