Abstract
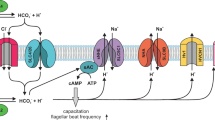
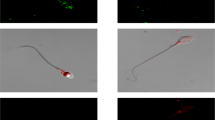
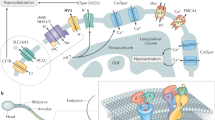
Thanks to a worrying decrease in male fertility, understanding how sperm 'work' is a matter both of interest and great importance. Sperm of all animals detect various environmental cues. The 'behavioural' and physiological responses of sperm must be specific, appropriate and correctly timed. Strangely, in a cell with few organelles and minimal cytoplasmic volume, internal Ca2+ concentration, [Ca2+]i, regulates almost all these activities. How does such a simple cell achieve this — and is it as simple as it seems?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hull, M. G. et al. Population study of causes, treatment, and outcome of infertility. BMJ 291, 1693–1697 (1985).
Sharpe, R. M. & Irvine, D. S. How strong is the evidence of a link between environmental chemicals and adverse effects on human reproductive health? BMJ 328, 447–451 (2004).
http://www.hfea.gov.uk/cps/rde/xbcr/SID-3F57D79B-12E4689D/hfea/facts_and_figures.pdf
Andersen, A. N. et al. High frequencey of sub-optimal semen quality in an unselected population of young men. Hum. Reprod. 15, 366–372 (2005).
Maher, E. R. Imprinting and assisted reproductive technology. Hum. Mol. Genet. 14, R133–R138 (2005).
Hansen, M., Bower, C., Milne, E., de Klerk, N. & Kurinczuk, J. J. Assisted reproductive technologies and the risk of birth defects — a systematic review. Hum. Reprod. 20, 328–338 (2005).
Strauss, J. F., 3rd & Kafrissen, M. Waiting for the second coming. Nature 432, 43–45 (2004).
Sitruk-Ware, R. New progestagens for contraceptive use. Hum. Reprod. 12, 169–178 (2006).
Holden, C. A. et al. Sexual activity, fertility and contraceptive use in middle-aged and older men: Men in Australia, Telephone Survey (MATeS). Hum. Reprod. 20, 3429–3434 (2005).
Barratt, C. L. & Publicover, S. J. Interaction between sperm and zona pellucida in male fertility. Lancet 358, 1660–1662 (2001).
Forti, G. et al. Effects of progesterone on human spermatozoa: clinical implications. Ann. Endocrinol. 60, 107–110 (1999).
Carlson, A. E. et al. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl Acad. Sci. USA 100, 14864–14868 (2003).
Liu de, Y. & Baker, H. W. Disordered zona pellucida-induced acrosome reaction and failure of in vitro fertilization in patients with unexplained infertility. Fertil. Steril. 79, 74–80 (2003).
Okunade, G. W. et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem. 279, 33742–33750 (2004).
Schuh, K. et al. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 279, 28220–28226 (2004).
Neill, A. T. & Vacquier, V. D. Ligands and receptors mediating signal transduction in sea urchin spermatozoa. Reproduction 127, 141–149 (2004).
Eisenbach, M. & Giojalas, L. C. Sperm guidance in mammals — an unpaved road to the egg. Nature Rev. Mol. Cell. Biol. 7, 276–285 (2006).
Kaupp, U. B., Hildebrand, E. & Weyand, I. Sperm chemotaxis in marine invertebrates-molecules and mechanisms. J. Cell Physiol. 208, 487–494 (2006).
Suarez, S. S. & Pacey, A. A. Sperm transport in the female reproductive tract. Hum. Reprod. 12, 23–37 (2006).
Marquez, B. & Suarez, S. S. Different signaling pathways in bovine sperm regulate capacitation and hyperactivation. Biol. Reprod. 70, 1626–1633 (2004).
Yanagimachi, R. The Physiology of Reproduction (Raven Press, New York, 1994).
Saunders, C. M. et al. PLC ζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129, 3533–3544 (2002).
Ainsworth, C. Cell biology: the secret life of sperm. Nature 436, 770–771 (2005).
Rassoulzadegan, M. et al. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature 441, 469–474 (2006).
Gur, Y. & Breitbart, H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 20, 411–416 (2006).
Breitbart, H. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Mol. Cell. Endocrinol. 187, 139–144 (2002).
Hess, K. C. et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 9, 249–259 (2005).
Evans, J. P. & Florman, H. M. The state of the union: the cell biology of fertilization. Nature Cell Biol. 4, S57–S63 (2002).
Suarez, S. S. & Ho, H. C. Hyperactivation of mammalian sperm. Cell. Mol. Biol. 49, 351–356 (2003).
Harper, C. V., Barratt, C. L. & Publicover, S. J. Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. J. Biol. Chem. 279, 46315–46325 (2004).
Darszon, A. et al. Calcium channels and Ca2+ fluctuations in sperm physiology. Int. Rev. Cytol. 243, 79–172 (2005).
Darszon, A. et al. Sperm channel diversity and functional multiplicity. Reproduction 131, 977–988 (2006).
Jimenez-Gonzalez, C., Michelangeli, F., Harper, C. V., Barratt, C. L. & Publicover, S. J. Calcium signalling in human spermatozoa: a specialized 'toolkit' of channels, transporters and stores. Hum. Reprod. 12, 253–267 (2006).
Wennemuth, G., Babcock, D. F. & Hille, B. Calcium clearance mechanisms of mouse sperm. J. Gen. Physiol. 122, 115–128 (2003).
Gunaratne, H. J., Neill, A. T. & Vacquier, V. D. Plasma membrane calcium ATPase is concentrated in the head of sea urchin spermatozoa. J. Cell. Physiol. 207, 413–419 (2006).
Walensky, L. D. & Snyder, S. H. Inositol 1,4,5-trisphosphate receptors selectively localized to the acrosomes of mammalian sperm. J. Cell Biol. 130, 857–869 (1995).
Kuroda, Y., Kaneko, S., Yoshimura, Y., Nozawa, S. & Mikoshiba, K. Are there inositol 1,4,5-triphosphate (IP3) receptors in human sperm? Life Sci. 65, 135–143 (1999).
Minelli, A., Allegrucci, C., Rosati, R. & Mezzasoma, I. Molecular and binding characteristics of IP3 receptors in bovine spermatozoa. Mol. Reprod. Dev. 56, 527–533 (2000).
Ho, H. C. & Suarez, S. S. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biol. Reprod. 68, 1590–1596 (2003).
Naaby-Hansen, S. et al. Co-localization of the inositol 1,4,5-trisphosphate receptor and calreticulin in the equatorial segment and in membrane bounded vesicles in the cytoplasmic droplet of human spermatozoa. Mol. Hum. Reprod. 7, 923–933 (2001).
Marquez, B., Ignotz, G. & Suarez, S. S. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: Release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Dev Biol. doi: 10.1016/j.ydbio.006.11 (2006)
Gunaratne, H. J. & Vacquier, V. D. Evidence for a secretory pathway Ca2+-ATPase in sea urchin spermatozoa. FEBS Lett. 580, 3900–3904 (2006).
Suarez, S. S. & Dai, X. Intracellular calcium reaches different levels of elevation in hyperactivated and acrosome-reacted hamster sperm. Mol. Reprod. Dev. 42, 325–333 (1995).
Meizel, S. The sperm, a neuron with a tail: 'neuronal' receptors in mammalian sperm. Biol. Rev. Camb. Philos. Soc. 79, 713–732 (2004).
Kirichok, Y., Navarro, B. & Clapham, D. E. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439, 737–740 (2006).
Qi, H., Moran, M. M. et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl Acad. Sci. USA 104, 1219–1223 (2007).
Carlson, A. E. et al. Identical phenotypes of CatSper1 and CatSper2 null sperm. J. Biol. Chem. 280, 32238–32244 (2005).
Wood, C. D., Darszon, A. & Whitaker, M. Speract induces calcium oscillations in the sperm tail. J. Cell Biol. 161, 89–101 (2003).
Bohmer, M. et al. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J. 24, 2741–2752 (2005).
Wood, C. D., Nishigaki, T., Furuta, T., Baba, S. A. & Darszon, A. Real-time analysis of the role of Ca2+ in flagellar movement and motility in single sea urchin sperm. J. Cell Biol. 169, 725–731 (2005).
Strünker, T. et al. A K+-selective cGMP-gated ion channel controls chemosensation of sperm. Nature Cell Biol. 8, 1149–1154 (2006).
Spehr, M. et al. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299, 2054–2058 (2003).
Spehr, M. et al. Particulate adenylate cyclase plays a key role in human sperm olfactory receptor-mediated chemotaxis. J. Biol. Chem. 279, 40194–40203 (2004).
Neuhaus, E. M., Mashukova, A., Barbour, J., Wolters, D. & Hatt, H. Novel function of β-arrestin2 in the nucleus of mature spermatozoa. J. Cell Sci. 119, 3047–3056 (2006).
Fukuda, N., Yomogida, K., Okabe, M. & Touhara, K. Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility. J. Cell Sci. 117, 5835–5845 (2004).
Wennemuth, G., Carlson, A. E., Harper, A. J. & Babcock, D. F. Bicarbonate actions on flagellar and Ca2+ -channel responses: initial events in sperm activation. Development 130, 1317–1326 (2003).
Castanas, E. Membrane steroid receptor signaling in normal and neoplastic cells. Mol. Cell. Endocrinol. 246, 76–82 (2006).
Zhu, Y., Rice, C. D., Pang, Y., Pace, M. & Thomas, P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Natl Acad. Sci. USA 100, 2231–2236 (2003).
Thomas, P. et al. Binding characteristics, hormonal regulation and identity of the sperm membrane progestin receptor in Atlantic croaker. Steroids 70, 427–433 (2005).
Bedu-Addo, K., Barratt, C. L., Kirkman-Brown, J. C. & Publicover, S. J. Patterns of [Ca2+]i mobilization and cell response in human spermatozoa exposed to progesterone. Dev. Biol. 302, 324–332 (2007).
De Blas, G. et al. The intraacrosomal calcium pool plays a direct role in acrosomal exocytosis. J. Biol. Chem. 277, 49326–49331 (2002).
Fukami, K. et al. Phospholipase Cδ4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. J. Cell Biol. 161, 79–88 (2003).
Herrick, S. B. et al. The acrosomal vesicle of mouse sperm is a calcium store. J. Cell. Physiol. 202, 663–671 (2005).
Conner, S. J. et al. Understanding the physiology of pre-fertilisation events in the human spermatozoa – a necessary prerequisite to developing rational therapy. Reprod. 63, 237–256 (2007).
Teves, M. E. et al. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil Steril. 86, 745–749 (2006).
Harper, C. V. & Publicover, S. J. Reassessing the role of progesterone in fertilization — compartmentalized calcium signalling in human spermatozoa? Hum. Reprod. 20, 2675–2680 (2005).
Jaiswal, B. S., Tur-Kaspa, I., Dor, J., Mashiach, S. & Eisenbach, M. Human sperm chemotaxis: is progesterone a chemoattractant? Biol. Reprod. 60, 1314–1319 (1999).
Kumar, P. & Meizel, S. Nicotinic acetylcholine receptor subunits and associated proteins in human sperm. J. Biol. Chem. 280, 25928–25935 (2005).
Dajas-Bailador, F. & Wonnacott, S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 25, 317–334 (2004).
Bray, C., Son, J. H. & Meizel, S. Acetylcholine causes an increase of intracellular calcium in human sperm. Mol. Hum. Reprod. 11, 881–889 (2005).
Bray, C., Son, J. H., Kumar, P. & Meizel, S. Mice deficient in CHRNA7, a subunit of the nicotinic acetylcholine receptor, produce sperm with impaired motility. Biol. Reprod. 73, 807–814 (2005).
Patrat, C. et al. Zona pellucida from fertilised human oocytes induces a voltage-dependent calcium influx and the acrosome reaction in spermatozoa, but cannot be penetrated by sperm. BMC Dev. Biol. 6, 59 (2006).
Jungnickel, M. K., Marrero, H., Birnbaumer, L., Lemos, J. R. & Florman, H. M. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nature Cell Biol. 3, 499–502 (2001).
Leypold, B. G. et al. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl Acad. Sci. USA 99, 6376–6381 (2002).
Stowers, L., Holy, T. E., Meister, M., Dulac, C. & Koentges, G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science 295, 1493–1500 (2002).
Domino, S. E. & Garbers, D. L. The fucose-sulfate glycoconjugate that induces an acrosome reaction in spermatozoa stimulates inositol 1,4,5-trisphosphate accumulation. J. Biol. Chem. 263, 690–695 (1988).
Gonzalez-Martinez, M. T. et al. A sustained increase in intracellular Ca2+ is required for the acrosome reaction in sea urchin sperm. Dev. Biol. 236, 220–229 (2001).
Hirohashi, N. & Vacquier, V. D. Store-operated calcium channels trigger exocytosis of the sea urchin sperm acrosomal vesicle. Biochem. Biophys. Res. Commun. 304, 285–292 (2003).
Travis, A. J. & Kopf, G. S. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J. Clin. Invest. 110, 731–736 (2002).
Nipper, R. W., Jones, B. H., Gerton, G. L. & Moss, S. B. Protein domains govern the intracellular distribution of mouse AKAP4. Biol. Reprod. 75, 189–196 (2006).
Matzuk, M. M. & Lamb, D. J. Genetic dissection of mammalian fertility pathways. Nature Cell Biol. 4, S41–S49 (2002).
Miyamoto, T. et al. Azoospermia in patients heterozygous for a mutation in SYCP3. Lancet 362, 1714–1719 (2003).
Baker, M. A., Witherdin, R., Hetherington, L., Cunningham-Smith, K. & Aitken, R. J. Identification of post-translational modifications that occur during sperm maturation using difference in two-dimensional gel electrophoresis. Proteomics 5, 1003–1012 (2005).
Pixton, K. L. et al. Sperm proteome mapping of a patient who experienced failed fertilization at IVF reveals altered expression of at least 20 proteins compared with fertile donors: case report. Hum. Reprod. 19, 1438–1447 (2004).
Johnston, D. S., Wooters, J., Kopf, G. S., Qiu, Y. & Roberts, K. P. Analysis of the human sperm proteome. Ann. N. Y. Acad. Sci. 1061, 190–202 (2005).
Kim, K. S. & Gerton, G. L. Differential release of soluble and matrix components: evidence for intermediate states of secretion during spontaneous acrosomal exocytosis in mouse sperm. Dev. Biol. 264, 141–152 (2003).
O'Toole, C. M., Arnoult, C., Darszon, A., Steinhardt, R. A. & Florman, H. M. Ca2+ entry through store-operated channels in mouse sperm is initiated by egg ZP3 and drives the acrosome reaction. Mol. Biol. Cell. 11, 1571–1584 (2000).
Harper, C. et al. Secretory pathway Ca2+-ATPase (SPCA1) Ca2+ pumps, not SERCAs, regulate complex [Ca2+]i signals in human spermatozoa. J. Cell Sci. 118, 1673–1685 (2005).
Acknowledgements
Our thanks to B. Michell, S. Clarke and M. Publicover for their comments and suggestions on preliminary versions of this paper. We gratefully acknowledge financial support from the Wellcome Trust, Medical Research Council (MRC) and the Biotechnology and Biological Sciences Research Council (BBSRC).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Publicover, S., Harper, C. & Barratt, C. [Ca2+]i signalling in sperm — making the most of what you've got. Nat Cell Biol 9, 235–242 (2007). https://doi.org/10.1038/ncb0307-235
Issue Date:
DOI: https://doi.org/10.1038/ncb0307-235
This article is cited by
-
Identification of ITPR1 gene as a novel target for hsa-miR-34b-5p in non-obstructive azoospermia: a Ca2+/apoptosis pathway cross-talk
Scientific Reports (2023)
-
Microfluidics facilitating the use of small extracellular vesicles in innovative approaches to male infertility
Nature Reviews Urology (2023)
-
The influence of the female reproductive tract and sperm features on the design of microfluidic sperm-sorting devices
Journal of Assisted Reproduction and Genetics (2022)
-
UV filters in matched seminal fluid-, urine-, and serum samples from young men
Journal of Exposure Science & Environmental Epidemiology (2021)
-
Structure-based redesigning of pentoxifylline analogs against selective phosphodiesterases to modulate sperm functional competence for assisted reproductive technologies
Scientific Reports (2021)