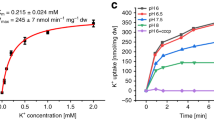
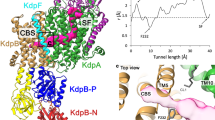
Abstract
Cellular potassium import systems play a fundamental role in osmoregulation, pH homeostasis and membrane potential in all domains of life. In bacteria, the kdp operon encodes a four-subunit potassium pump that maintains intracellular homeostasis, cell shape and turgor under conditions in which potassium is limiting1. This membrane complex, called KdpFABC, has one channel-like subunit (KdpA) belonging to the superfamily of potassium transporters and another pump-like subunit (KdpB) belonging to the superfamily of P-type ATPases. Although there is considerable structural and functional information about members of both superfamilies, the mechanism by which uphill potassium transport through KdpA is coupled with ATP hydrolysis by KdpB remains poorly understood. Here we report the 2.9 Å X-ray structure of the complete Escherichia coli KdpFABC complex with a potassium ion within the selectivity filter of KdpA and a water molecule at a canonical cation site in the transmembrane domain of KdpB. The structure also reveals two structural elements that appear to mediate the coupling between these two subunits. Specifically, a protein-embedded tunnel runs between these potassium and water sites and a helix controlling the cytoplasmic gate of KdpA is linked to the phosphorylation domain of KdpB. On the basis of these observations, we propose a mechanism that repurposes protein channel architecture for active transport across biomembranes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Greie, J. C. The KdpFABC complex from Escherichia coli: a chimeric K+ transporter merging ion pumps with ion channels. Eur. J. Cell Biol. 90, 705–710 (2011)
Epstein, W. The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 75, 293–320 (2003)
Diskowski, M., Mikusevic, V., Stock, C. & Hänelt, I. Functional diversity of the superfamily of K+ transporters to meet various requirements. Biol. Chem. 396, 1003–1014 (2015)
Epstein, W., Whitelaw, V. & Hesse, J. A K+ transport ATPase in Escherichia coli. J. Biol. Chem. 253, 6666–6668 (1978)
Buurman, E. T., Kim, K. T. & Epstein, W. Genetic evidence for two sequentially occupied K+ binding sites in the Kdp transport ATPase. J. Biol. Chem. 270, 6678–6685 (1995)
Dorus, S., Mimura, H. & Epstein, W. Substrate-binding clusters of the K+-transporting Kdp ATPase of Escherichia coli investigated by amber suppression scanning mutagenesis. J. Biol. Chem. 276, 9590–9598 (2001)
Siebers, A. & Altendorf, K. Characterization of the phosphorylated intermediate of the K+-translocating Kdp-ATPase from Escherichia coli. J. Biol. Chem. 264, 5831–5838 (1989)
Irzik, K. et al. The KdpC subunit of the Escherichia coli K+-transporting KdpB P-type ATPase acts as a catalytic chaperone. FEBS J. 278, 3041–3053 (2011)
Gassel, M., Möllenkamp, T., Puppe, W. & Altendorf, K. The KdpF subunit is part of the K+-translocating Kdp complex of Escherichia coli and is responsible for stabilization of the complex in vitro. J. Biol. Chem. 274, 37901–37907 (1999)
Møller, J. V., Olesen, C., Winther, A. M. & Nissen, P. The sarcoplasmic Ca2+-ATPase: design of a perfect chemi-osmotic pump. Q. Rev. Biophys. 43, 501–566 (2010)
Cao, Y. et al. Crystal structure of a potassium ion transporter, TrkH. Nature 471, 336–340 (2011)
Vieira-Pires, R. S., Szollosi, A. & Morais-Cabral, J. H. The structure of the KtrAB potassium transporter. Nature 496, 323–328 (2013)
Hänelt, I. et al. Gain of function mutations in membrane region M2C2 of KtrB open a gate controlling K+ transport by the KtrAB system from Vibrio alginolyticus. J. Biol. Chem. 285, 10318–10327 (2010)
Fendler, K., Dröse, S., Epstein, W., Bamberg, E. & Altendorf, K. The Kdp-ATPase of Escherichia coli mediates an ATP-dependent, K+-independent electrogenic partial reaction. Biochemistry 38, 1850–1856 (1999)
Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000)
Bramkamp, M., Altendorf, K. & Greie, J. C. Common patterns and unique features of P-type ATPases: a comparative view on the KdpFABC complex from Escherichia coli (Review). Mol. Membr. Biol. 24, 375–386 (2007)
Zhou, Y., Morais-Cabral, J. H., Kaufman, A. & MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature 414, 43–48 (2001)
Gassel, M., Siebers, A., Epstein, W. & Altendorf, K. Assembly of the Kdp complex, the multi-subunit K+-transport ATPase of Escherichia coli. Biochim. Biophys. Acta 1415, 77–84 (1998)
Sitsel, O. et al. Structure and function of Cu(I)- and Zn(II)-ATPases. Biochemistry 54, 5673–5683 (2015)
Miller, A. N. & Long, S. B. Crystal structure of the human two-pore domain potassium channel K2P1. Science 335, 432–436 (2012)
Payandeh, J., Gamal El-Din, T. M., Scheuer, T., Zheng, N. & Catterall, W. A. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature 486, 135–139 (2012)
Rasaiah, J. C., Garde, S. & Hummer, G. Water in nonpolar confinement: from nanotubes to proteins and beyond. Annu. Rev. Phys. Chem. 59, 713–740 (2008)
Cao, Y. et al. Gating of the TrkH ion channel by its associated RCK protein TrkA. Nature 496, 317–322 (2013)
Olesen, C., Sørensen, T. L., Nielsen, R. C., Møller, J. V. & Nissen, P. Dephosphorylation of the calcium pump coupled to counterion occlusion. Science 306, 2251–2255 (2004)
Puppe, W., Siebers, A. & Altendorf, K. The phosphorylation site of the Kdp-ATPase of Escherichia coli: site-directed mutagenesis of the aspartic acid residues 300 and 307 of the KdpB subunit. Mol. Microbiol. 6, 3511–3520 (1992)
Bramkamp, M. & Altendorf, K. Single amino acid substitution in the putative transmembrane helix V in KdpB of the KdpFABC complex of Escherichia coli uncouples ATPase activity and ion transport. Biochemistry 44, 8260–8266 (2005)
Becker, D., Fendler, K., Altendorf, K. & Greie, J. C. The conserved dipole in transmembrane helix 5 of KdpB in the Escherichia coli KdpFABC P-type ATPase is crucial for coupling and the electrogenic K+-translocation step. Biochemistry 46, 13920–13928 (2007)
Liu, S. & Lockless, S. W. Equilibrium selectivity alone does not create K+-selective ion conduction in K+ channels. Nat. Commun. 4, 2746 (2013)
Toyoshima, C., Nomura, H. & Tsuda, T. Lumenal gating mechanism revealed in calcium pump crystal structures with phosphate analogues. Nature 432, 361–368 (2004)
Møller, J. V., Lenoir, G., Le Maire, M., Juul, B. S. & Champeil, P. Proteolytic studies on the transduction mechanism of sarcoplasmic reticulum Ca2+-ATPase: common features with other P-type ATPases. Ann. NY Acad. Sci. 986, 82–89 (2003)
Walderhaug, M. O. et al. KdpD and KdpE, proteins that control expression of the kdpABC operon, are members of the two-component sensor-effector class of regulators. J. Bacteriol. 174, 2152–2159 (1992)
Siebers, A. & Altendorf, K. The K+-translocating Kdp-ATPase from Escherichia coli. Purification, enzymatic properties and production of complex- and subunit-specific antisera. Eur. J. Biochem. 178, 131–140 (1988)
Laimins, L. A., Rhoads, D. B. & Epstein, W. Osmotic control of kdp operon expression in Escherichia coli. Proc. Natl Acad. Sci. USA 78, 464–468 (1981)
Doublié, S. Production of selenomethionyl proteins in prokaryotic and eukaryotic expression systems. Methods Mol. Biol. 363, 91–108 (2007)
Warren, G. B., Toon, P. A., Birdsall, N. J. M., Lee, A. G. & Metcalfe, J. C. Reversible lipid titrations of the activity of pure adenosine triphosphatase-lipid complexes. Biochemistry 13, 5501–5507 (1974)
Wheeler, M. J., Russi, S., Bowler, M. G. & Bowler, M. W. Measurement of the equilibrium relative humidity for common precipitant concentrations: facilitating controlled dehydration experiments. Acta Crystallogr. F Struct. Biol. Cryst. Commun. 68, 111–114 (2012)
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010)
Sheldrick, G. M. Experimental phasing with SHELXC/D/E: combining chain tracing with density modification. Acta Crystallogr. D Biol. Crystallogr. 66, 479–485 (2010)
Bricogne, G., Vonrhein, C., Flensburg, C., Schiltz, M. & Paciorek, W. Generation, representation and flow of phase information in structure determination: recent developments in and around SHARP 2.0. Acta Crystallogr. D Biol. Crystallogr. 59, 2023–2030 (2003)
Abrahams, J. P. & Leslie, A. G. Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr. D Biol. Crystallogr. 52, 30–42 (1996)
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010)
Gourdon, P. et al. Crystal structure of a copper-transporting PIB-type ATPase. Nature 475, 59–64 (2011)
van der Laan, M., Gassel, M. & Altendorf, K. Characterization of amino acid substitutions in KdpA, the K+-binding and -translocating subunit of the KdpFABC complex of Escherichia coli. J. Bacteriol. 184, 5491–5494 (2002)
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010)
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007)
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010)
Petrek, M. et al. CAVER: a new tool to explore routes from protein clefts, pockets and cavities. BMC Bioinformatics 7, 316 (2006)
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011)
Eser, E., Can, T. & Ferhatosmanog˘lu, H. Div-BLAST: diversification of sequence search results. PLoS ONE 9, e115445 (2014)
Pei, J., Kim, B. H. & Grishin, N. V. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 36, 2295–2300 (2008)
Hu, G. B., Rice, W. J., Dröse, S., Altendorf, K. & Stokes, D. L. Three-dimensional structure of the KdpFABC complex of Escherichia coli by electron tomography of two-dimensional crystals. J. Struct. Biol. 161, 411–418 (2008)
Heitkamp, T., Böttcher, B. & Greie, J. C. Solution structure of the KdpFABC P-type ATPase from Escherichia coli by electron microscopic single particle analysis. J. Struct. Biol. 166, 295–302 (2009)
Heitkamp, T. et al. K+-translocating KdpFABC P-type ATPase from Escherichia coli acts as a functional and structural dimer. Biochemistry 47, 3564–3575 (2008)
Schrader, M. et al. Replacement of glycine 232 by aspartic acid in the KdpA subunit broadens the ion specificity of the K(+)-translocating KdpFABC complex. Biophys. J. 79, 802–813 (2000)
Bertrand, J., Altendorf, K. & Bramkamp, M. Amino acid substitutions in putative selectivity filter regions III and IV in KdpA alter ion selectivity of the KdpFABC complex from Escherichia coli. J. Bacteriol. 186, 5519–5522 (2004)
Sørensen, T. L., Møller, J. V. & Nissen, P. Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science 304, 1672–1675 (2004)
Jensen, A. M., Sørensen, T. L., Olesen, C., Møller, J. V. & Nissen, P. Modulatory and catalytic modes of ATP binding by the calcium pump. EMBO J. 25, 2305–2314 (2006)
Toyoshima, C. & Mizutani, T. Crystal structure of the calcium pump with a bound ATP analogue. Nature 430, 529–535 (2004)
Bond, C. S. & Schüttelkopf, A. W. ALINE: a WYSIWYG protein-sequence alignment editor for publication-quality alignments. Acta Crystallogr. D Biol. Crystallogr. 65, 510–512 (2009)
Pedersen, B. P., Buch-Pedersen, M. J., Morth, J. P., Palmgren, M. G. & Nissen, P. Crystal structure of the plasma membrane proton pump. Nature 450, 1111–1114 (2007)
Andersson, M. et al. Copper-transporting P-type ATPases use a unique ion-release pathway. Nat. Struct. Mol. Biol. 21, 43–48 (2014)
Laursen, M., Yatime, L., Nissen, P. & Fedosova, N. U. Crystal structure of the high-affinity Na+K+-ATPase–ouabain complex with Mg2+ bound in the cation binding site. Proc. Natl Acad. Sci. USA 110, 10958–10963 (2013)
Acknowledgements
The authors acknowledge beamline 23IDB/IDD at the Advanced Photon Source—Argonne National Laboratory, where all the X-ray data were collected, as well as the National Synchrotron Light Source and the Stanford Synchrotron Radiation Light Source. We also thank W. Epstein and D. Sauer for discussions, W. Epstein for the plasmid and bacterial strain for expression, and J. Deng and T. Neubert for mass spectrometry. This work was supported by funding from NIH R01 GM108043 and NIH T32 GM088118 to D.L.S., the European Research Council (grant agreement No. 637372), the Danish Council for Independent Research (grant agreement no. DFF-4002-00052) and an AIAS fellowship to B.P.P.
Author information
Authors and Affiliations
Contributions
C.-S.H. produced protein, grew crystals, collected data, ran crystallographic analysis programs to solve the structure, interpreted the structure and wrote the manuscript. B.P.P. devised data collection strategies, analysed crystallographic data, interpreted the structure and wrote the manuscript. D.L.S. collected data, analysed sequences, interpreted the structure and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Figure 1 Structure determination.
a, Crystals had plate-like morphology with a substantial level of birefringence. b, Anomalous density maps from Hg (red mesh at 5σ) and seleno-methionine (blue mesh at 3σ) were used to identify positions of cysteine and methionine residues, respectively. This image shows one of the three KdpFABC complexes in the asymmetric unit with the locations of seven Hg atoms and 44 Se atoms superimposed on the ribbon model of the KdpFABC complex (colours as in Fig. 1). Considering all three complexes in the asymmetric unit, we were able to identify a total of 21 Hg sites and 132 Se sites, which were a powerful constraint for building the model before refinement. c, Elution profiles from size-exclusion chromatography using multiple detectors to quantify the size of the complex. Traces are shown for multiple angle laser light scattering (LS), refractive index (RI) and absorption at 280 nm (UV). On the basis of these data, we determined the sizes of the protein-detergent complex (PDC), the KdpFABC complex and the detergent micelle. Given the expected Mr of 160 kD, these data are consistent with a monomeric KdpFABC complex. d, 2mFo−DFc map densities at 1.5σ are superimposed with the refined model at 2.9 Å resolution to demonstrate the quality of the fit. The green chain corresponds to the N terminus of KdpA (upper right corner) and helices from the second M1PM2 repeat. The purple chain corresponds to the transmembrane domain of KdpC (far left) and part of the periplasmic domain (top). A series of bulky aromatic residues towards the periplasmic side of the KdpC helix unambiguously establishes its orientation in the membrane. e, mFo−DFc difference density (purple at 6.5σ) was present in the S3 site of the KdpA selectivity filter. The anomalous signal from K (cyan at 3.8σ) is consistent with the presence of a K+ ion at this site. f, mFo−DFc difference density (purple at 4.5σ) next to the unwound portion of M4 in KdpB was consistent with the presence of a water molecule at this canonical ion binding site in P-type ATPases. No anomalous signal from K was seen at this site. g, Three KdpFABC complexes compose the asymmetric unit and are coloured in shades of red, green and blue. Intermolecular contacts can be seen between the periplasmic domain of KdpC and the cytoplasmic face of KdpA. At the current resolution, the three complexes have identical structures. Although previous results suggest that KdpFABC forms a dimer51,52,53, we did not observe dimer formation either during purification or within our crystal lattice. This result suggests that dimerization may depend on specific conditions, such as the choice of detergent or the presence of a lipid bilayer. h, The three complexes have been coloured according to the temperature factor using a standard colour palette moving from blue (lowest B factor) to red (highest B factor). KdpA appears to be the best ordered and peripheral regions of the N- and A-domains display the most flexibility. i, In addition to the four protein subunits, two detergent molecules (OG) and two lipid molecules (DMPC) were modelled into densities at the periphery of the structure for each complex.
Extended Data Figure 2 Mutations that lower the apparent K+ affinity of KdpFABC.
a, b, Two orthogonal views of KdpA showing the locations of mutations within the selectivity filter that lower the apparent affinity of the pump for K+. The sites of the mutants are indicated by a red sphere at the Cα position; the selectivity filter is coloured yellow and the coupling helix (D3M2) is coloured pink. Although Lys substitutions generally dominate the reported mutations5,6,43,54,55, the selectivity filter has a range of other substitutions that are additionally shown to have an effect. c, d, Additional sites of K+ affinity mutants populate the pore helix in MPM2–4, which presumably support the architecture of the selectivity filter. These are predominantly Lys substitutions, suggesting that the addition of positive charge in this vicinity also plays a role in lowering the affinity5,6.
Extended Data Figure 3 Comparison of selectivity filters from a K+ channel and SKT transporters.
a, KcsA is an archetypal K+ channel and the 2.0 Å resolution structure (1K4C)17 shows four K+ sites within the selectivity filter at sites designated S1–S4. These sites are presumed to be alternatively occupied by K+ and water molecules, but differences have been masked by crystallographic averaging. Additional, partially hydrated K+ ions are seen in cytoplasmic and periplasmic vestibules at either end of the filter. b, TrkH is more closely related to KdpA and displays the gating loop at the cytoplasmic end of the selectivity filter (pink). The crystal structure (3PJZ)11 shows a single K+ ion in the S3 position and the filter is considerably more disordered towards its periplasmic end, perhaps accounting for the lack of further ions. c, KdpA has intermediate order in the selectivity filter. A K+ ion occupies the S3 site and the side chain of Arg116, which was substituted for Gln in our construct, appears to occupy the S1 site. Like TrkH, a gating loop is evident from the kinked helix (pink) in the third M1PM2 repeat. d, The cage of oxygen atoms that coordinate the K+ ions in the selectivity filter of KcsA. The strict fourfold symmetry of this channel produces a very regular geometry. e, The analogous oxygen atoms in the TrkH structure show considerably more distortion of this cage and complete absence of coordination at the S1 sites. f, The coordination cage for KdpA has intermediate order with reasonable evidence for sites at S2–S4.
Extended Data Figure 4 Comparison of KdpB and SERCA1a.
a, In the P domain of KdpB (blue), there is no evidence for phosphorylation of the catalytic Asp307, which is in a binding network with Lys499, Asp522 and Thr309. The location of the nucleotide binding pocket is illustrated by some of the conserved residues (Glu348, Phe377 and Thr429) expected to interact with ATP in the N domain (red). b, Overlay of the N domains of KdpB (red) and SERCA1a (cyan, 1T5T)56 with bound nucleotide (ADP-AlF4). This overlay illustrates how, if the nucleotide were bound to the corresponding site in KdpB, the phosphate groups would clash with the conserved catalytic loop (DKTGTLT in green). The TGES162 loop of KdpB is coloured pink. Taken together, the Asp307 binding network and the positional decoupling of the A domain from the rest of the structure indicate that KdpB is in an E1 state in which Asp307 remains unphosphorylated and the N domain nucleotide binding site is empty15,56,57,58. c, The binding site for Ca(II) (green sphere)15 in the transmembrane domain of SERCA1a (1T5T) consists of main chain carbonyls from the unwound M4 helix near the canonical Pro308 as well as side chain densities from the neighbouring M6 helix (Asn796 and Asp800). The second Ca2+ ion in the Ca(I) site is not shown, but involves additional ligands from the M5 helix. d, The water site (red sphere) in the transmembrane domain of KdpB involves residues homologous to SERCA1a in a very similar configuration: namely, the main chain carbonyls on M4 near Pro264 and the side chain of Asn624. The side chains of Asp583 and Lys586 from M5 are also nearby, which align with Asn768 and Glu771 in SERCA1a that bind Ca(I).
Extended Data Figure 5 Serine phosphorylation of KdpFABC.
a, Evidence for the phosphoserine comes from an extra density seen both in the 2mFo−DFc map (grey at 1.2σ) and the mFo−DFc difference density (purple at 4σ) from a refined model that lacks the phosphate group. b, To confirm the presence of the phosphoserine, ESI–LC–MS/MS was performed on KdpB isolated by SDS–PAGE from a crystal. Peptides harbouring phosphorylated and unphosphorylated Ser162 were separated by FPLC and their relative abundance is represented by these elution profiles for peptides with m/z = 1,010.26 (unphosphorylated) or m/z = 1,030.25 (phosphorylated) (z = 4). The sequence of these peptides, in which the cysteine was modified by iodoacetamide, is shown above, as determined by MS2. c, The cytoplasmic domains of KdpB adopt a unique position in our structure compared to other P-type ATPases owing to a salt bridge between the phosphorylated Ser162 in the A domain (yellow) and residues Lys357 and Arg363 of the N domain (red). This interaction is likely to prevent N domain movements, and the close proximity of the P domain to the N domain prevents nucleotide binding, suggesting that the current structure represents an autoinhibited state. d, The level of Ser162 phosphorylation inversely correlated with the ATPase activity of KdpFABC. The absolute level of phosphoenzyme was not determined owing to a lack of a fully dephosphorylated or fully phosphorylated enzyme preparation for calibration of the mass spectrometer detection system; nevertheless, the inverse correlation between the relative levels of Ser162 phosphorylation and ATPase activity supports the inhibitory nature of this modification. Although this serine phosphorylation was observed in samples isolated directly from the bacteria, its physiological relevance is uncertain, especially given that Lys357 and Arg363 are poorly conserved amongst KdpFABC systems (Extended Data Fig. 9). Footnotes for this table: 1apparent percentage of phosphopeptide from mass spectrometry does not reflect lower detection efficiency of the phosphopeptide; 2ATPase assay was determined in the presence of 50 mM KCl; 3200 units of protein phosphatase from bacteriophage lambda (LPP) were incubated with 20 μg KdpFABC under the conditions indicated.
Extended Data Figure 6 Sequence alignment of KdpA with other SKT transporters TrkH and KtrB.
This structure-based alignment was done by the promals3d server50 using PDB depositions 4J7C for KtrB12 and 3PJZ for TrkH11. Sequence conservation is shown by colour with red being most conserved and green least conserved. Secondary structure based on KdpA is shown with the colouring scheme used in Fig. 1c. The four M1PM2 motifs are denoted D1–D4, with S indicating the selectivity filter. The red star indicates the Q116R mutation and red triangles indicate residues implicated in coupling with KdpB, namely Glu370 and Arg493, which reside at the cytoplasmic side of the selectivity filter (Fig. 2c), and Arg400, which forms a salt bridge with the P domain (Fig. 3b). This figure and Extended Data Figs 7, 8, 9 were made with ALINE59.
Extended Data Figure 7 Sequence alignment of KdpB with other P-type ATPases.
This structure-based alignment was done by the promals3d server using PDB depositions 1SU4 for the calcium pump (SERCA)15, 5KSD for the plasma membrane proton pump (AHA2)60, 4BYG for the copper pump (CopA)61 and 4HYT for the Na,K-ATPase62. Sequence conservation is shown by the colour scheme with red being most conserved and green least conserved. Secondary structure elements correspond to KdpB with the same colour scheme as in Fig. 1. The red stars indicate the catalytic Asp307 and other residues involved in the hydrogen bonding network shown in Extended Data Fig. 4a (Thr309, Lys399 and Asp522). The red diamonds indicate residues at the water site along M4 and M6 shown in Extended Data Fig. 4d (Pro264 and Asn624). Red triangles indicate residues implicated in coupling with KdpA, namely Asp583 and Lys586 (Extended Data Fig. 4d) in the transmembrane domain as well as Asp300 and Asp302, which interact with cytoplasmic loops from KdpA (Fig. 3b). The red circles indicate phosphorylated Ser162 and Lys357/Arg363 from the N domain, which form salt bridges to the phosphate (Extended Data Fig. 5c).
Extended Data Figure 8 Sequence alignment of KdpA from a diverse group of bacteria.
Selection of the sequences was done with the divblast server49 and alignment using clustal omega48. Secondary structure corresponds to that observed for KdpA (Fig. 1). Pink stars indicate residues reported in ref. 5 and pink diamonds indicate residues reported in ref. 6 to increase the apparent Kd for K+ to 0.3 mM or higher. Red triangles indicate residues implicated in coupling with KdpB, namely Glu370 and Arg493 at the cytoplasmic end of the selectivity filter (Fig. 2c) and Arg400, which forms a salt bridge to the P domain of KdpB (Fig. 3b). Blue triangles indicate residues forming hydrogen bonds to the periplasmic domain of KdpC.
Extended Data Figure 9 Sequence alignment of KdpB from a diverse group of bacteria.
Selection of sequences was done with the divblast server49 and alignment using clustal omega48. Secondary structure corresponds to that observed for KdpB (Fig. 1). Red stars indicate residues that interact with the catalytic Asp307 in our structure. Red triangles indicate residues that interact with cytoplasmic loops from KdpA (Asp300 and Asp302). Red circles indicate Ser162, which is phosphorylated in our structure, and the residues in the N domain that engage this phosphate in a salt bridge (Lys357 and Arg363).
Rights and permissions
About this article
Cite this article
Huang, CS., Pedersen, B. & Stokes, D. Crystal structure of the potassium-importing KdpFABC membrane complex. Nature 546, 681–685 (2017). https://doi.org/10.1038/nature22970
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature22970
This article is cited by
-
KdpD is a tandem serine histidine kinase that controls K+ pump KdpFABC transcriptionally and post-translationally
Nature Communications (2024)
-
Enhancing coevolutionary signals in protein–protein interaction prediction through clade-wise alignment integration
Scientific Reports (2024)
-
Potassium Solubilizing Microorganisms as Potential Biofertilizer: A Sustainable Climate-Resilient Approach to Improve Soil Fertility and Crop Production in Agriculture
Journal of Plant Growth Regulation (2024)
-
Wie Bakterien die Aufnahme von Kaliumionen regulieren
BIOspektrum (2022)
-
What do we know about osmoadaptation of Yersinia pestis?
Archives of Microbiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.