Abstract
Behavioural responses to temperature are critical for survival, and animals from insects to humans show strong preferences for specific temperatures1,2. Preferred temperature selection promotes avoidance of adverse thermal environments in the short term and maintenance of optimal body temperatures over the long term1,2, but its molecular and cellular basis is largely unknown. Recent studies have generated conflicting views of thermal preference in Drosophila, attributing importance to either internal3 or peripheral4 warmth sensors. Here we reconcile these views by showing that thermal preference is not a singular response, but involves multiple systems relevant in different contexts. We found previously that the transient receptor potential channel TRPA1 acts internally to control the slowly developing preference response of flies exposed to a shallow thermal gradient3. We now find that the rapid response of flies exposed to a steep warmth gradient does not require TRPA1; rather, the gustatory receptor GR28B(D) drives this behaviour through peripheral thermosensors. Gustatory receptors are a large gene family, widely studied in insect gustation and olfaction, and are implicated in host-seeking by insect disease vectors5,6,7, but have not previously been implicated in thermosensation. At the molecular level, GR28B(D) misexpression confers thermosensitivity upon diverse cell types, suggesting that it is a warmth sensor. These data reveal a new type of thermosensory molecule and uncover a functional distinction between peripheral and internal warmth sensors in this tiny ectotherm reminiscent of thermoregulatory systems in larger, endothermic animals2. The use of multiple, distinct molecules to respond to a given temperature, as observed here, may facilitate independent tuning of an animal’s distinct thermosensory responses.
Similar content being viewed by others
Main
Thermal preference is an important body temperature control mechanism from insects to humans1,2. In Drosophila two sets of warmth-sensing neurons (activated above ∼25 °C) have been proposed to control thermal preference: the anterior cell (AC) neurons3, located inside the head, and the hot cell (HC) neurons4, located peripherally in the arista (Fig. 1a). However, different studies suggest conflicting cellular and molecular mechanisms for thermal preference control. At the cellular level, primary importance has been attributed to either internal3 or peripheral4 warmth sensors. At the molecular level, the internal AC neurons sense warmth via TrpA1 (ref. 3), which encodes a warmth-activated transient receptor potential (TRP) channel3,8, whereas the peripheral HC neurons seem to be TrpA1-independent4. To clarify the mechanisms of thermal preference, we sought to discover the molecular basis of HC neuron function.
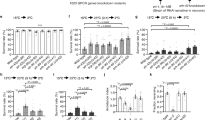
a, Head. eso, oesophagus. b, Arista. c–g, HC-GAL4;UAS-nls:GFP (in which UAS denotes upstream activation sequence and nls denotes nuclear localization signal) (c), CC-GAL4;UAS-nls:GFP (d), Gr28b.d-GAL4;UAS-nls:GFP (e), HC-GAL4;Gr28b.d-GAL4;UAS-nls:GFP (f), CC-GAL4;Gr28b.d-GAL4;UAS-nls:GFP (g). Arrowheads, cell bodies; carats, processes. Scale bar, 15 μm. h, UAS-TNT (n = 22), HC-GAL4 (n = 15), HC>TNT (n = 29), TrpA1GAL4/+ (n = 9), TrpA1GAL4>TNT (n = 9). i, Wild type (n = 12), TrpA1ins (n = 29). j, Gr28b. Arrows denote isoform-specific start sites. Green line, Gr28b.d-GAL4 promoter. k, Wild type (n = 12), Gr28bMi (n = 13), Gr28bMi/Df(Gr28b) (in which Df denotes deficiency; n = 6), revertant (n = 10), rescue (n = 8). l, Gr28bMi;Gr28b(D)-GAL4 (n = 14), Gr28bMi;UAS-Gr28b(D) (n = 10), Gr28bMi;Gr28b(D)>Gr28b(D) (n = 7), Gr28bMi;HC-GAL4 (n = 9), Gr28bMi;HC>Gr28b(D) (n = 12). Data are mean ± s.e.m.; n, number of independent assays. **Significantly different from wild type (k) or UAS and Gal4 controls (h, l) (Tukey’s honestly significant difference test (HSD), α = 0.01).
The arista contains six neurons9: three warmth-responsive HC neurons (which can be labelled using cell-specific Gal4 expression in the HC-GAL4 strain4) and three cool-responsive (cold cell; CC) neurons (labelled in the CC-GAL4 strain4) (Fig. 1b–d). Three unidentified cells in the arista have been reported to express Gr28b.d-GAL4, a transgene in which promoter sequences upstream of the gustatory receptor GR28B(D) control Gal4 expression10. We found that these Gr28b.d-GAL4-expressing cells resembled thermoreceptors, with cell bodies near the arista base and thin processes in the shaft (Fig. 1e). To determine the thermoreceptor subset labelled, Gr28b.d-GAL4 was combined with each thermoreceptor-specific Gal4. Gr28b.d-GAL4 plus HC-GAL4 labelled three neurons (Fig. 1f, n = 5), whereas Gr28b.d-GAL4 plus CC-GAL4 labelled six neurons (Fig. 1g, n = 5), indicating that Gr28b.d-GAL4 is expressed in the HC neurons. Although in situ hybridization was unsuccessful (common for gustatory receptors5), GR28B(D) transcripts were robustly detected in dissected antennae/aristae from wild-type, but not Gr28b mutant, animals by reverse transcriptase PCR (RT–PCR) (Supplementary Fig. 1), demonstrating expression in this tissue.
Gustatory receptors are a large family of seven transmembrane proteins present in invertebrates7, with 68 members in Drosophila melanogaster11 (Supplementary Fig. 2). Insects also contain multiple gustatory receptor-related odorant receptors (62 in D. melanogaster11). Gustatory receptors and odorant receptors form a gene family distinct from, and apparently unrelated to, the G-protein-coupled receptor superfamily7. Gustatory receptors and odorant receptors have been studied extensively as chemoreceptors for sweet and bitter tastants, food odours, carbon dioxide and other chemicals5,6,7, but have not previously been implicated in thermosensation. We examined gustatory receptor involvement in thermosensation using a two-temperature choice assay12, exposing flies for 1 min to a steep thermal gradient (initially >5 °C per cm) created using tubes of ∼25.5 and ∼31.0 °C air (a preferred and an increased-but-innocuous temperature, respectively) separated by 1 cm. Flies normally prefer the cooler tube, a behaviour termed ‘rapid negative thermotaxis’ (Fig. 1h, i). Consistent with a previous report4, inhibiting HC neurons by cell-specific expression of tetanus toxin light chain (TNT), a vesicle release inhibitor13, using HC-GAL4 strongly reduced such behaviour (Fig. 1h). In agreement with the importance of HC neurons, and in addition to previous studies14, third antennal segment/arista removal strongly reduced this behaviour, whereas ablating other tissues expressing HC-GAL4 and Gr28b.d-GAL4 did not (Supplementary Figs 3–5). By contrast, inhibiting AC neurons by TNT expression using TrpA1GAL4, a Gal4 knock-in at the TrpA1 locus15, had no effect (Fig. 1h). (This manipulation disrupted a previously reported AC-dependent thermosensory behaviour3 (Supplementary Fig. 6).) These data indicate that rapid negative thermotaxis depends on the peripheral HC warmth sensors.
To probe the molecular basis of rapid negative thermotaxis, we first examined its dependence on TrpA1, which is required for AC neuron warmth-sensing3. Consistent with the TrpA1-independence of HC neuron thermosensitivity4, a strong loss-of-function TrpA1 mutation did not affect this behaviour (Fig. 1i). By contrast, strong loss-of-function mutations in the gene encoding GR28B(D) eliminated the response; Gr28b mutants distributed nearly equally between ∼25.5 °C and ∼31.0 °C (Fig. 1k). The defect was specific: excising the transposon in the Gr28bMi allele restored thermotaxis (Fig. 1k), and both a Gr28b-containing genomic transgene and Gr28b(D) complementary DNA expression rescued the mutant (Fig. 1k, l). We also attempted rescue by expressing cDNAs for the other Drosophila GR28 family members10,11 (four other Gr28b isoforms (Fig. 1j) and Gr28a11,12) under Gr28b.d-GAL4 control. Although a negative result could reflect a failure to be properly expressed, only Gr28b(E) yielded significant rescue (Supplementary Fig. 7). However, endogenous Gr28b(E) transcripts were not detected in the antenna/arista (Supplementary Fig. 1), consistent with a previous analysis indicating that GR28B(E) is not expressed there10. Together, these data demonstrate that rapid negative thermotaxis depends not on TrpA1, but on Gr28b, consonant with the specific dependence of this behaviour on HC neuron function (Fig. 1h). Notably, cell-specific GR28B(D) expression using HC-GAL4 strongly rescued the Gr28b mutant (Fig. 1l), indicating that GR28B(D) function in the HC thermosensors is sufficient to restore rapid negative thermotaxis.
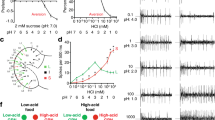
To test whether GR28B(D) might act as a thermosensor, we examined whether it conferred warmth-sensitivity when ectopically expressed. Unlike controls, flies broadly expressing GR28B(D) under Actin5C-GAL4 control were incapacitated when heated to 37 °C for 3 min, recovering when returned to 23 °C (Fig. 2a and Supplementary Video 1). This dramatic effect suggested that GR28B(D) might promote warmth-responsive neuronal activation. We showed previously that ectopic expression of the warmth-activated cation channel TRPA1(B), a product of Drosophila TrpA1, renders fly chemosensors warmth-responsive16. Like TRPA1(B), chemosensor expression of GR28B(D) (using Gr5a-GAL4) conferred robust warmth-responsiveness (Fig. 2b). We examined the behavioural consequences of such GR28B(D) expression. When chemically activated, sweet-responsive chemosensors promote proboscis extension5,6. When GR28B(D) was expressed in these cells, strong proboscis extension was elicited by warming to ∼32 °C (Fig. 2c). This ability to confer warmth-responsiveness is consistent with GR28B(D) acting as a warmth sensor.
a, Top, flies before and after warming. Bottom, knockdown quantification (n = 3 independent assays per genotype, >10 flies per assay). Ectopic Gr28b(D) denotes Actin5C-GAL4;UAS-Gr28b(D). b, Gustatory bristle responses to warming. Top, extracellular recording traces. Bottom, average spike rate from gustatory bristles during warming, after subtracting electrolyte-only baseline. Gr5a-GAL4 (n = 6) bristles; UAS-Gr28b(D) (n = 9), Gr5a>Gr28b(D) (n = 17). c, Frequency of proboscis extension upon warming to ∼32 °C (n = 32 flies per genotype). Data are mean ± s.e.m. **Significantly different from UAS and Gal4 alone controls (Tukey’s HSD, α = 0.01).
Whether GR28B(D) requires sensory neuron-specific cofactors was examined in the neuromuscular system. Unlike controls, motor neurons expressing GR28B(D) (using OK371-GAL4) triggered warmth-responsive excitatory junction potentials at the neuromuscular junction (Fig. 3a). Thus, GR28B(D)-mediated warmth-responsiveness does not require sensory neuron-specific cofactors. The threshold for GR28B(D)-dependent muscle stimulation was 26.0 ± 0.3 °C (± s.e.m., n = 12), just above TRPA1(B)’s ∼25 °C threshold in this system17, indicating that both molecules mediate responses to innocuous warming.
a, Top, muscle response to warming in OK371>Gr28b(D) animals. Bottom, excitatory junction potentials (EJP) during temperature course. OK371-GAL4 (n = 12) muscles; UAS-Gr28b(D) (n = 13), OK371>Gr28b(D) (n = 9). b, Currents in voltage-clamped motor neurons upon warming. OK371-GAL4 (n = 5) motor neurons; UAS-Gr28b(D) (n = 5), OK371>Gr28b(D) (n = 7). c, Arrhenius plot of warmth-responsive current of OK371>Gr28b(D) motor neuron in panel b. d, Currents in voltage-clamped muscles upon warming. Mhc-GAL4 (n = 3) muscles; UAS-Gr28b(D) (n = 3), Mhc>Gr28b(D) (n = 7). Data are mean ± s.e.m. **Significantly different from UAS and Gal4 alone controls (Tukey’s HSD, α = 0.01).
To quantify the thermosensitivity of GR28B(D)-dependent responses, currents were monitored using whole-cell patch-clamp electrophysiology. Unlike controls, voltage-clamped motor neurons expressing GR28B(D) exhibited warmth-responsive inward currents (Fig. 3b). The response’s temperature coefficient (Q10, fold change in current per 10 °C change) was calculated by Arrhenius analysis18 (Fig. 3c). GR28B(D)-dependent currents were highly thermosensitive (Q10 of 25 ± 5 (s.e.m., n = 7)), similar to mammalian neurons expressing thermosensitive TRP channels18. Substituting N-methyl-d-glucamine (NMDG)+ for Na+ in the extracellular solution eliminated heat-responsiveness, consistent with cation channel activation (n = 3; Supplementary Fig. 8).
The potential dependence of GR28B(D) on neuron-specific cofactors was tested in muscle. Although control muscles voltage-clamped at −60 mV exhibited modest warmth-responsive outward currents (Fig. 3d), muscles expressing GR28B(D) (using Mhc-GAL4) exhibited robust warmth-responsive inward currents (Fig. 3d). The ability of GR28B(D) to confer warmth sensitivity across diverse cell types supports the hypothesis that GR28B(D) acts as a molecular thermoreceptor. It further suggests GR28B(D) as a new class of tool for thermogenetic neuronal activation, adding to the TRP-based toolbox currently used in Drosophila19.
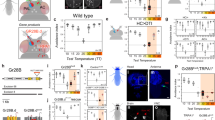
Although GR28B(D) resembles TRPA1(B) in conferring warmth-sensitivity3,16,17, these two proteins have distinct functions in the fly, with only Gr28b controlling rapid negative thermotaxis (Fig. 1). TrpA1 was found previously to control the slowly developing thermal preference response of flies on a shallow, broad thermal gradient (∼0.5 °C per cm, 18–32 °C)3. We tested the contribution of Gr28b to this long-term body temperature selection behaviour. As reported previously3, TrpA1 mutants selected unusually warm temperatures after 30 min on the gradient, with many accumulating at ≥28 °C (Fig. 4a). By contrast, strong loss-of-function Gr28b mutants behaved indistinguishably from wild type (Fig. 4a). This neatly distinguishes Gr28b and TrpA1, with the former controlling rapid negative thermotaxis and the latter long-term body temperature selection.
a, Left, fly distribution across gradient (30 min). Right, flies in ≥28 °C and ≥30 °C regions. Wild type (n = 11), Gr28bMi/Df(Gr28b) (n = 8), TrpA1ins (n = 17). b, Rapid negative thermotaxis. Gr28bMi;Gr28b.d-GAL4 (n = 14), Gr28bMi;UAS-Gr28b(D) (n = 10), Gr28bMi;UAS-TrpA1(A) (n = 8), Gr28bMi;UAS-TrpA1(B) (n = 9); Gr28bMi;Gr28b.d>Gr28b(D) (n = 7), Gr28bMi;Gr28b.d>TrpA1(A) (n = 9); Gr28bMi;Gr28b.d>TrpA1(B) (n = 9) (GAL4 alone, UAS-Gr28b(D) alone and Gr28b(D) rescue data from Fig. 1l.) c, Data presented as in a. Wild type (n = 11), TrpA1ins/TrpA1GAL4 (n = 12), TrpA1ins,UAS-Gr28b(D) (n = 11), TrpA1ins/TrpA1GAL4>Gr28b(D) (n = 14), TrpA1ins,UAS-TrpA1(B) (n = 6), TrpA1ins/TrpA1GAL4>TrpA1(B) (n = 5). Data are mean ± s.e.m.; n, number of independent assays. Letters denote statistically distinct groups (Tukey’s HSD, α = 0.01).
These findings reconcile previously disparate views of Drosophila thermosensation3,4 by demonstrating that thermal preference is not a singular behaviour, but involves multiple systems relevant in different contexts. It suggests a model in which Gr28b, acting peripherally, controls rapid responses to ambient temperature jumps, whereas TrpA1, acting internally, controls responses to sustained temperature increases reaching the core. In the arista, Gr28b could experience ambient temperature fluctuations in advance of core changes, eliciting rapid avoidance. Such behaviour could be critical for a tiny animal in which ambient and core temperatures equalize rapidly1. The dispensability of Gr28b for responses on the shallow gradient (Fig. 4a) could relate to observations in other insects where peripheral thermoreceptors respond more to temperature fluctuations than absolute values20. The fly’s reliance on distinct sensors for distinct aspects of thermal preference is reminiscent of complex thermosensory systems of larger, endothermic animals2. In the fly, these warmth-responsive pathways potentially converge in the brain, where both sets of sensors innervate overlapping regions4.
Finally, we tested whether Gr28b and TrpA1 were uniquely suited to their roles in the fly. Although TrpA1 was normally not required for rapid negative thermotaxis (Fig. 1i), when expressed in the arista using Gr28b.d-GAL4, TRPA1(B) significantly rescued the Gr28b mutant defect (Fig. 4b). (As expected, a less thermosensitive TrpA1 isoform, TRPA1(A)16, did not rescue the defect (Fig. 4b).) Conversely, although Gr28b was not normally required for slowly developing thermal preference on the shallow gradient (Fig. 4a), GR28B(D) expression under TrpA1GAL4 control significantly rescued the TrpA1 mutant defect (Fig. 4c). Thus, when their expression is manipulated appropriately, GR28B(D) and TRPA1(B) can act in the same cells and support the same behaviours, indicating fundamental functional similarities.
Although studied extensively, the mechanisms of gustatory receptor action are not fully resolved7. Gustatory receptors have been reported to act as cation channels7,21 and via G-proteins22. Whether GR28B(D) acts by either mechanism remains unknown. Although attempts to study GR28B(D) in heterologous cells (including Xenopus laevis oocytes and HEK cells; L.N., T. Lauer, P. Taneja, S. Nelson and P.A.G., unpublished observations) were unsuccessful, the ability of GR28B(D) to confer warmth-responsiveness upon diverse cell types argues against a requirement for cell-type-specific cofactors in the fly. Gr28b has been implicated in responses to strong illumination23. This seems to be unrelated to GR28B(D)-dependent thermosensation, as Gr28b-dependent photosensors are unresponsive to innocuous warming23 and appear to express other Gr28b isoforms10. GR28B(D)-expressing muscles were not light-responsive (n = 4, Supplementary Fig. 9).
Previous studies have demonstrated the importance of TRP channels in Drosophila thermosensation1, stimulating interest in their potential involvement in warmth-dependent host-seeking by insect disease vectors24. This work raises the possibility that gustatory receptors, including GR28 receptors in disease vectors such as tsetse flies and mosquitoes (Supplementary Fig. 2), regulate thermosensation more broadly. GR28B(D) adds to a growing list of highly thermosensitive membrane proteins including not only TRPs, but the mammalian ANO1 chloride channel25 and calcium-channel regulator STIM1 (ref. 26). The presence of exceptional thermosensitivity in diverse proteins may facilitate temperature-responsive modulation of diverse physiological responses. Furthermore, using multiple molecules to mediate behavioural responses to similar temperatures may facilitate independent tuning of distinct thermosensory responses.
Methods Summary
Fly strains
Gr28b, TrpA1, HC-GAL4 and CC-GAL4 strains were previously described3,4,10,15,16,23,27. Df(Gr28b) is Df(2L)Exel7031 (Bloomington Stock Center). To control for transposon position effects, all UAS-Gr28 transgenes were inserted at the same landing site, attp2, by site-specific transgenesis16.
Behaviour and physiology
Two-temperature rapid negative thermotaxis assay was as described12, except tube temperatures were 25.5 ± 0.3 °C and 31.0 ± 0.5 °C (± s.d.), ≥15 flies per trial. Thermal preference assay was as described3,12, with 20–60 flies (2–5 days old) per trial. For proboscis extension, female flies (1–5 days old) were starved overnight with water, then glued to glass slides and heated16. Flies were given three 5-s heat presentations at 5-s intervals. Physiology is detailed in methods.
Molecular biology
Transgenic fly creation and RT–PCR were performed as described16. RT–PCR primers straddled splice junctions to minimize genomic DNA amplification. Three independent tissue preparations gave similar results.
Phylogeny
As gustatory receptor sequence diversity creates the potential for alignment ambiguities, phylogeny was created using BAli-Phy28, which performs simultaneous Bayesian inference of alignment and phylogeny. Further details provided in Methods.
Online Methods
Fly strains
Gr28b, TrpA1, HC-GAL4 and CC-GAL4 strains were previously described3,4,10,15,16,23,27. Df(Gr28b) is Df(2L)Exel7031 (Bloomington Stock Center). To control for transposon position effects, all UAS-Gr28 transgenes were inserted at the same landing site, attp2, by site-specific transgenesis16. UAS-Gr28b(B) was created from expressed sequence tag clone IP03356 (DGRC stock no. 1623277). Alternative amino termini of UAS-Gr28b(A), UAS-Gr28b(C) and UAS-Gr28b(D) were amplified from cDNA with N-terminal primers (UAS-Gr28b(A): 5′-CCGGAATTCATGATCCGCTGCGGATTGGAC-3′; UAS-Gr28b(C): 5′-CCGGAATTCATGGACATTGAAATGGCCAAGG-3′; and UAS-Gr28b(D): 5′-CCGGAATTCATGTCATTTTACTTTTGCGAA-3′) and common primer (5′-TCCGCAGGATCCTTGGTTACAATGG-3′). UAS-Gr28b(E) was amplified from genomic DNA with primers 5′-CCGGAATTCATGTGGCTCCTTAGGCGATCGG-3′ and 5′-TCCGCAGGATCCTTGGTTACAATGG-3′. The first intron of Gr28b(E) was deleted by PCR (5′-GCACTTAACGAGGTGTTGAAGAACC-3′ and 5′-GGTTCTTCAACACCTCGTTAAGTGC-3′). UAS-Gr28a transgene was amplified from genomic DNA with primers 5′-CCGGAATTCATGGCCTTTAAGTTGTGGGAG-3′ and 5′-TCCCCTCGAGGTATATATAATTTTAATCAATCG-3′. The introns were deleted by PCR (first intron: 5′-TATCCTGCAGGATTTCGTTTAACATACTAA-3′ and 5′-TTAGTATGTTAAACGAAATCCTGCAGGATA-3′; second intron: 5′-GGCAGCACCAGTAATCGTAAAAATCAGTGTG-3′ and 5′-CACACTGATTTTTACGATTACTGGTGCTGCC-3′). All clones were sequenced to confirm that no mutations were introduced. TRPA1(A) resembles dTRPA1-D29, but contains 20 additional N-terminal amino acids. TRPA1(B) corresponds to dTRPA1-A29.
Behaviour and electrophysiology
Two-temperature rapid negative thermotaxis assay was performed as described12, except that tube temperatures were 25.5 ± 0.3 °C and 31.0 ± 0.5 °C (± s.d.), ≥15 flies per trial. Ablations were performed with Ultra Fine Clipper Scissors (Fine Science Tools) on ice-anaesthetized 1–4-day-old white Canton-S flies. Recovery was 1 h to 2 days. For rapid (1 min) phototaxis, all flies were collected after thermotaxis assay and re-tested using same apparatus but clear-walled tubes (BD Falcon) in a dark-lined box exposed on one side to ultraviolet light (ULTRA-LUM, no. UVA-16). Thermal preference assay was performed as described3,12, with 20–60 flies (2–5 days old) per trial. For proboscis extension, female flies (1–5 days old) were starved overnight with water, then glued to glass slides and heated16. Flies were given three 5-s heat presentations at 5-s intervals.
Extracellular recordings of gustatory neurons were performed as described16. At least three animals and six bristles were examined per genotype.
Neuromuscular junction potentials were recorded by current clamp from muscle 6 with 3 M KCl-filled intracellular electrodes (20–30 MΩ) in 0.4 mM Ca2+ HL3.1, using an Axoclamp2B (Molecular Devices) and a Digidata1322A (Molecular Devices), recording at 5 kHz with pClamp8 (Molecular Devices). Muscles had −45 mV or lower resting potentials. Perfusate was heated with SC-20/CL100 cooler/controller (Warner Instruments) and temperature monitored with bath thermistor (Warner Instruments) or IT-23 thermocouple (Physitemp) connected to 80TK Thermocouple (Fluke).
Muscle currents were recorded by two-electrode voltage clamp at −60 mV from muscle 6 as above, but using 0 Ca2+ HL3.1 solution with 0.5 mM EGTA, 12 mM MgCl2, 100 μM quinidine and 1 mM 4-AP, and 3 M KCl-filled voltage-sensing (10–15 MΩ) and 3 M CH3COOK-filled current-passing (5–10 MΩ) electrodes. For light responsiveness, experiments were as above with 0 Ca2+ HL3.1 with 0.5 mM EGTA, 4 mM MgCl2, 100 μM quinidine and 1 mM 4-AP. 30–50 s dark baseline (<0.1 μW mm−2 at 400 nm) was recorded, followed by two ∼30-s pulses from halogen source at indicated intensities, followed by heat ramp. Intensity was measured using PM100 light meter (Thor) with 400 nm wavelength correction. Intensity (in mW mm−2) across wavelengths measured: pulse 1: 1.4 at 400 nm, 0.25 at 488 nm, 0.17 at 577 nm, 0.02 at 700 nm; pulse 2: 4.3 at 400 nm, 1.02 at 488 nm, 0.75 at 577 nm, 0.1 at 700 nm. Intensities are minimum estimates; meter was ∼2 mm further from source than preparation.
Motor neuron currents were recorded at −60 mV by whole-cell patch clamp with Multiclamp700A amplifier (Molecular Devices) and patch pipettes (3.5–4 MΩ). External solution was a nominally Ca2+-free modified A solution (in mM: 118 NaCl, 2 KCl, 4 MgCl2, 5 Trehalose, 45.5sucrose, 5 HEPES), 290 mOsm, pH 7.1–7.2, with 0.15 μM tetrodotoxin to limit spiking. The internal solution (in mM: 2 NaCl, 130 K-gluconate, 0.1 CaCl2, 2 MgCl2, 1 EGTA, 0.2 Na-GTP, 10 HEPES) adjusted to 285 mOsm with glucose, and pH 7.1–7.2 with KOH. Dorsal motor neurons below nerve cord sheath were visualized with ×40 water immersion objective and exposed using 0.75% (w/v) protease (type XIV, Sigma) in modified A solution. For ion substitution, after initial heating, perfusion was changed to nominally Ca2+-free external Modified A solution of the same osmolarity with NaCl replaced by equimolar NMDG and HCl. After 5 min NMDG solution perfusion, preparation was reheated. Perfusion was than reverted to nominally Ca2+-free modified A solution. After 5 min, a third heat ramp was recorded. Trace plotting and analysis performed in Matlab. All neuromuscular physiology used female third instar larvae.
The data presented reflect biological replicates as noted in each sample’s n. Sample sizes were chosen to reliably reveal robust distinctions among samples. No blinding or randomization was used. Nonparametric analysis (Kruskal–Wallis/Steel–Dwass All Pairs test (JMP10, SAS)) yielded results similar to Tukey’s HSD.
Molecular biology
Transgenic fly creation and RT–PCR were performed as described16. RT–PCR primers straddled splice junctions to minimize genomic DNA amplification. Three independent tissue preparations gave similar results. Primers for RT–PCR reactions: Gr28a forward primer: 5′-CAGCACCAGTAATCGTAAAAATC-3′; Gr28a reverse primer: 5′-TATGTTAAACGAAATCCTGCAGG-3′; Gr28b(A) forward primer: 5′-AACGTTTGCGAAGTCCTGTC-3′; Gr28b(B) forward primer: 5′-GCTGTGATTTATACGTCGGC-3′; Gr28b(C) forward primer: 5′-CTGTCATCTACCTGACTGCC-3′; Gr28b(D) forward primer: 5′-TTCCTCTGCAGCAGCATTCG-3′; Gr28b(A), Gr28b(B), Gr28b(C) and Gr28b(D) common reverse primer: 5′-TCCTGTATAATCTCCGCAGG-3′; Gr28b(D) reverse primer (used in Supplementary Fig. 1a): 5′-CTTGACCTCAGCACTTTTGG-3′; Gr28b(E) forward primer: 5′-GGCCCCGCTGATCGTGAAA-3′; Gr28b(E) reverse primer: 5′-GCACTTAACGAGGTGTTGAAG-3′.
Phylogeny
As gustatory receptor sequence diversity creates the potential for alignment ambiguities, phylogeny was created using BAli-Phy28, which performs simultaneous Bayesian inference of alignment and phylogeny. The analysis was performed using the RS07 insertion/deletion model30, LG substitution matrix31, estimating equilibrium amino acid frequencies, with gamma distributed rate variation (four categories). Two independent chains were run until the average standard deviation of split frequencies (ASDSF) and potential scale reduction factor (PSRF) based on width of 80% credible intervals (PSRF-80%CI) criteria fell below 0.01 and 1.01, respectively.
Change history
19 August 2013
The sentence starting: ‘To quantify the thermosensitivity...’ was corrected in the HTML.
References
Garrity, P. A., Goodman, M. B., Samuel, A. D. & Sengupta, P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 24, 2365–2382 (2010)
Flouris, A. D. Functional architecture of behavioural thermoregulation. Eur. J. Appl. Physiol. 111, 1–8 (2011)
Hamada, F. N. et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008)
Gallio, M., Ofstad, T. A., Macpherson, L. J., Wang, J. W. & Zuker, C. S. The coding of temperature in the Drosophila brain. Cell 144, 614–624 (2011)
Thorne, N., Chromey, C., Bray, S. & Amrein, H. Taste perception and coding in Drosophila. Curr. Biol. 14, 1065–1079 (2004)
Vosshall, L. B. & Stocker, R. F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533 (2007)
Silbering, A. F. & Benton, R. Ionotropic and metabotropic mechanisms in chemoreception: ‘chance or design’? EMBO Rep. 11, 173–179 (2010)
Viswanath, V. et al. Opposite thermosensor in fruitfly and mouse. Nature 423, 822–823 (2003)
Foelix, R. F., Stocker, R. F. & Steinbrecht, R. A. Fine structure of a sensory organ in the arista of Drosophila melanogaster and some other dipterans. Cell Tissue Res. 258, 277–287 (1989)
Thorne, N. & Amrein, H. Atypical expression of Drosophila gustatory receptor genes in sensory and central neurons. J. Comp. Neurol. 506, 548–568 (2008)
Robertson, H. M., Warr, C. G. & Carlson, J. R. Molecular evolution of the insect chemoreceptor gene superfamily in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 100 (suppl. 2). 14537–14542 (2003)
Sayeed, O. & Benzer, S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc. Natl Acad. Sci. USA 93, 6079–6084 (1996)
Sweeney, S. T., Broadie, K., Keane, J., Niemann, H. & O’Kane, C. J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron 14, 341–351 (1995)
Zars, T. Two thermosensors in Drosophila have different behavioral functions. J. Comp. Physiol. A 187, 235–242 (2001)
Kim, S. H. et al. Drosophila TRPA1 channel mediates chemical avoidance in gustatory receptor neurons. Proc. Natl Acad. Sci. USA 107, 8440–8445 (2010)
Kang, K. et al. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 481, 76–80 (2012)
Pulver, S. R., Pashkovski, S. L., Hornstein, N. J., Garrity, P. A. & Griffith, L. C. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J. Neurophysiol. 101, 3075–3088 (2009)
Vyklický, L. et al. Temperature coefficient of membrane currents induced by noxious heat in sensory neurones in the rat. J. Physiol. (Lond.) 517, 181–192 (1999)
Bernstein, J. G., Garrity, P. A. & Boyden, E. S. Optogenetics and thermogenetics: technologies for controlling the activity of targeted cells within intact neural circuits. Curr. Opin. Neurobiol. 22, 61–71 (2012)
Gingl, E., Hinterwirth, A. & Tichy, H. Sensory representation of temperature in mosquito warm and cold cells. J. Neurophysiol. 94, 176–185 (2005)
Sato, K., Tanaka, K. & Touhara, K. Sugar-regulated cation channel formed by an insect gustatory receptor. Proc. Natl Acad. Sci. USA 108, 11680–11685 (2011)
Liu, J. et al. elegans phototransduction requires a G protein-dependent cGMP pathway and a taste receptor homolog. Nature Neurosci. 13, 715–722 (2010)
Xiang, Y. et al. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 468, 921–926 (2010)
Wang, G. et al. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur. J. Neurosci. 30, 967–974 (2009)
Cho, H. et al. The calcium-activated chloride channel anoctamin 1 acts as a heat sensor in nociceptive neurons. Nature Neurosci. 15, 1015–1021 (2012)
Xiao, B., Coste, B., Mathur, J. & Patapoutian, A. Temperature-dependent STIM1 activation induces Ca2+ influx and modulates gene expression. Nature Chem. Biol. 7, 351–358 (2011)
Kang, K. et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 464, 597–600 (2010)
Suchard, M. A. & Redelings, B. D. BAli-Phy: simultaneous Bayesian inference of alignment and phylogeny. Bioinformatics 22, 2047–2048 (2006)
Zhong, L. et al. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP channel. Cell Rep. 1, 43–55 (2012)
Redelings, B. D. & Suchard, M. A. Incorporating indel information into phylogeny estimation for rapidly emerging pathogens. BMC Evol. Biol. 7, 40 (2007)
Le, S. Q. & Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 25, 1307–1320 (2008)
Acknowledgements
We thank the Garrity laboratory, C. B. Chien, L. Huang, R. Huey, E. Marder and G. Turrigiano for comments, H. Amrein, Y. Jan, C. Montell, C. Zuker and Bloomington Stock Center for strains, N. Donelson, M. Klein, A. Samuel, T. Lauer, P. Taneja, S. Nelson, Y. Yu, H. Bell, P. Sengupta, F. Baier and L. Vosshall for assistance. Supported by grants from National Institute of Mental Health (NIMH) (EUREKA R01 MH094721), National Institute of Neurological Disorders and Stroke (NINDS) (PO1 NS044232) and National Science Foundation (IOS-1025307) to P.A.G., National Institute of General Medical Sciences (NIGMS) (R01 GM054408) and NIMH (R01 MH067284) to L.C.G., NIGMS (R01 GM094468) to D.L.T. and NINDS National Research Service Award to V.C.P.
Author information
Authors and Affiliations
Contributions
L.N., P.B., A.M.L., L.C.G. and P.A.G. designed experiments. L.N. performed molecular genetics, behaviour and chemosensor electrophysiology. P.B. performed neuromuscular electrophysiology. E.C.C., A.M.L. and J.O.F. performed behaviour electrophysiology. V.C.P. performed chemosensor electrophysiology. D.L.T. and P.A.G. performed bioinformatics. L.N., P.B., D.L.T., L.C.G. and P.A.G. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-9. (PDF 10684 kb)
Warmth-triggered incapacitation of Actin>Gr28b(D) flies
Flies that express Gr28b(D) broadly (Actin-Gal4;UAS-Gr28b(D)) were temporarily incapacitated by warming, while control flies (containing only the Actin-Gal4 source or the UAS-Gr28b(D) transgene) were not. All flies were heated by submerging their vials in a 37˚C water bath for 180 seconds. Some frames were deleted as indicated. The video is accelerated 8-fold. (MOV 6085 kb)
Rights and permissions
About this article
Cite this article
Ni, L., Bronk, P., Chang, E. et al. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila. Nature 500, 580–584 (2013). https://doi.org/10.1038/nature12390
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12390
This article is cited by
-
Genetic atlas of hygro-and thermosensory cells in the vinegar fly Drosophila melanogaster
Scientific Reports (2023)
-
Pheromone sensing in Drosophila requires support cell-expressed Osiris 8
BMC Biology (2022)
-
Comparative analysis of temperature preference behavior and effects of temperature on daily behavior in 11 Drosophila species
Scientific Reports (2022)
-
Robustness and plasticity in Drosophila heat avoidance
Nature Communications (2021)
-
Thermoresponsive motor behavior is mediated by ring neuron circuits in the central complex of Drosophila
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.