Abstract
Many lines of evidence suggest that memory in the mammalian brain is stored with distinct spatiotemporal patterns1,2. Despite recent progresses in identifying neuronal populations involved in memory coding3,4,5, the synapse-level mechanism is still poorly understood. Computational models and electrophysiological data have shown that functional clustering of synapses along dendritic branches leads to nonlinear summation of synaptic inputs and greatly expands the computing power of a neural network6,7,8,9,10. However, whether neighbouring synapses are involved in encoding similar memory and how task-specific cortical networks develop during learning remain elusive. Using transcranial two-photon microscopy11, we followed apical dendrites of layer 5 pyramidal neurons in the motor cortex while mice practised novel forelimb skills. Here we show that a third of new dendritic spines (postsynaptic structures of most excitatory synapses) formed during the acquisition phase of learning emerge in clusters, and that most such clusters are neighbouring spine pairs. These clustered new spines are more likely to persist throughout prolonged learning sessions, and even long after training stops, than non-clustered counterparts. Moreover, formation of new spine clusters requires repetition of the same motor task, and the emergence of succedent new spine(s) accompanies the strengthening of the first new spine in the cluster. We also show that under control conditions new spines appear to avoid existing stable spines, rather than being uniformly added along dendrites. However, succedent new spines in clusters overcome such a spatial constraint and form in close vicinity to neighbouring stable spines. Our findings suggest that clustering of new synapses along dendrites is induced by repetitive activation of the cortical circuitry during learning, providing a structural basis for spatial coding of motor memory in the mammalian brain.
Similar content being viewed by others
Main
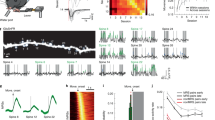
Spines are dendritic protrusions that contain all the essential components for postsynaptic signalling and are thus a good indicator of synaptic connectivity12,13. The clustered plasticity model suggests that neighbouring spines tend to transmit similar information to the postsynaptic neuron6,7. To investigate the formation and functional significance of spine clusters during learning, we trained thy1-YFP-H mice14 with a seed-reaching task15 and followed the dynamics of spines on apical dendrites of layer 5 (L5) pyramidal neurons in the motor cortex contralateral to the trained limb during different learning phases. We found that 32.5 ± 2.2% of new spines that formed during the acquisition phase of learning (early training, days 1–4) emerged in clusters; that is, two or more neighbouring new spines without interspersed existing spine(s) (Fig. 1a, b). Most such clusters (61 cases) comprised two contiguous new spines, and the other two clusters comprised three. In contrast, fewer new spine clusters emerged in untrained control mice over the same period (6.8 ± 4.6%, P < 0.01) or in trained mice during the consolidation phase of learning (late training, days 13–16; 7.4 ± 4.3%, P < 0.01; Fig. 1b). In addition to clustering of contiguous new spines, we observed a few cases in which two or more new spines formed in close vicinity to each other, but with up to three existing spines interspersed among them, as well as cases in which new filopodia clustered with new spines (Supplementary Fig. 1). We incorporated these cases in another set of analyses, in which a cluster was defined as a set of new spines/filopodia formed within 5 μm of each other, regardless of the presence or absence of existing spine(s) between them (Supplementary Information). These analyses again revealed that a significantly higher percentage of new spines clustered during early training, compared with that in controls or during late training (P < 0.01 for both cases; Supplementary Fig. 2). They also showed that filopodia only made a minor contribution to new protrusion clusters (Supplementary Fig. 3). More interestingly, among the new spines observed at the end of the acquisition phase (day 4), clustered new spines had a significantly higher survival rate than non-clustered ones (that is, individual new spines flanked by two existing spines) by training day 16 (P < 0.01), as well as 4 months after training stopped (P < 0.05; Fig. 1c). Together, our results reveal that motor learning induces coordinated formation of clustered synapses, which presumably belong to the same neuronal circuit and persist over time to encode motor information.
a, Repeated imaging of the same dendritic branch during motor learning reveals that a second new spine that formed between days 1 and 4 (red arrowhead) is located next to a stabilized new spine that had formed on day 1 (blue arrowhead). Scale bar, 1 μm. b, A higher percentage of new spines formed in clusters over 4 days during early training (n = 18 mice), compared with control (n = 7) and late training (n = 4). c, Clustered new spines observed on training day 4 have a higher survival rate than non-clustered counterparts by the end of the 16-day training (n = 6), as well as 4 months after training stops (n = 4). d, New spines formed on training day 1 are classified according to their fate and neighbouring spine formation. e, Spine head sizes of persistent clustered new spines increase between training days 1 and 4. f, Spine head sizes of persistent non-clustered new spines show no change between training days 1 and 4. Spine head size is quantified by the normalized spine head diameter, defined as the ratio of the spine head diameter to the adjacent dendritic shaft diameter. *P < 0.05, **P < 0.01. Error bars, s.e.m.
Perfection of a motor skill requires repeated practice, usually through multiple training sessions. We therefore sought to find out whether clustered new spines observed on training day 4 were formed within the same training session or across different sessions. We imaged the mice three times (on the day before training, and after 1 and 4 days of training), and found that among new spine clusters observed on training day 4, only 2.4% were composed of spines that formed together between training days 0 and 1. On the other hand, 43.9% of clusters were composed of spines formed between days 1 and 4, and the remaining 53.7% of clusters consisted of one spine formed between days 0 and 1 (the first new spine) and another spine formed between days 1 and 4 (the second new spine). Thus most new spine clusters emerged through recurrent training sessions. To determine how the formation of the second new spine in a cluster correlates with functional changes of the first new spine, we categorized first new spines into three groups based on their survival and neighbouring spine addition: transient new spines (formed on training day 1 but lost by day 4); persistent clustered new spines (formed on training day 1, survived until day 4, with an adjacent new spine formed between days 1 and 4); and persistent non-clustered new spines (formed on training day 1, survived until day 4, with no adjacent new spine formation) (Fig. 1d). As spine head size closely correlates with synaptic strength, we followed head sizes of first new spines over time. On training day 1, we found that head sizes of both transient and persistent new spines were significantly smaller than those of existing stable spines along the same dendrite (P < 0.001 for both cases, Supplementary Fig. 4). By training day 4, head sizes of persistent clustered new spines increased significantly (P < 0.01; Fig. 1e and Supplementary Fig. 5a), whereas head sizes of persistent non-clustered new spines remained comparable to day 1 (P > 0.2; Fig. 1f and Supplementary Fig. 5b). Because spine head size is a good proxy for synaptic strength, these data suggest that formation of the second new spines accompanies synaptic potentiation at first new spines during motor learning. It is consistent with previous reports that long-term potentiation at a single spine can facilitate formation and potentiation of neighbouring spines16,17.
Different sets of synapses have been shown to be involved in different motor tasks15. We therefore trained the same mice sequentially with two motor skills (cross-training) to determine if spines induced by different motor tasks cluster. Cross-training started with the reaching task on day 1 and then switched to the capellini-handling task, which also requires forelimb coordination, on days 2–4 (Fig. 2a and Supplementary Table 1). We found that 12.3 ± 0.4% new spines formed during the capellini-handling task between days 1 and 4, among which 28.4 ± 2.8% occurred in clusters (Fig. 2b–d). Both the spine formation rate and the percentage of clustered new spines were comparable to those in mice continuously trained with the reaching task (reach-only) (P > 0.5 in both cases), and were significantly higher than those in control mice over the same period of time (P < 0.01 in both cases, Fig. 2c, d). Thus, the capellini-handling task itself can induce clustered spine formation. However, only 3.3 ± 2.1% of capellini-handling-induced new spines clustered with reaching-induced new spines in cross-training. This contrasts with the outcome of reach-only training (13.8 ± 1.0%, P < 0.01, Fig. 2e), suggesting that new spines induced by different tasks have a low incidence of clustering with each other. To characterize further the task-specific nature of clustered spine formation, we housed animals in a motor enriched environment with daily change of motor challenges (Fig. 2a; see Methods). Motor enrichment also robustly enhanced spinogenesis: 13.7 ± 0.8% new spines formed between days 1 and 4, comparable to the percentages under reach-only and cross-training conditions (P > 0.1 for both cases). However, only 12.6 ± 1.1% of these new spines appeared in clusters, a percentage comparable to controls (P > 0.2, Fig. 2d) but significantly lower than that under reach-only or cross-training conditions (P < 0.01 for both cases). Together these data indicate that, whereas novelty in learning stimulates spinogenesis, repetitive activation of the same cortical circuit is crucial in clustered spine formation (Supplementary Fig. 6).
a, Timelines of reach-only, cross-training and motor enrichment experiments. b, Repeated imaging of the same dendritic branch revealed that two neighbouring new spines (arrowheads) formed between days 1 and 4 during cross-training. Scale bar, 1 μm. c, Higher percentages of new spines formed between days 1 and 4 in reach-only, cross-training and motor enrichment, compared with controls. d, Higher percentages of new spines formed in clusters between days 1 and 4 in reach-only and cross-training, compared with controls. e, A higher percentage of new spines that formed between days 1 and 4 clustered with new spines that had formed between days 0 and 1 in the reach-only condition, compared with controls. Number of mice examined: six control, nine reach-only, five cross-training and six motor enrichment. **P < 0.01, ***P < 0.001. Error bars, s.e.m.
The phenomenon of learning-induced, coordinated spinogenesis led us to investigate further the spatial distribution of new spines. We first examined the distance between a new spine (n) and its nearest existing spine (s) (Dn–s, Fig. 3a) in control mice. We then simulated Dn–s distribution under the null hypothesis that new spines form uniformly and independently along the dendrite (see Methods). Compared with simulation results, the median of measured values of Dn–s was significantly larger (Fig. 3b), and the cumulative probability distribution of measured values of Dn–s was shifted towards longer distances (Fig. 3c). These results suggest that new spines are not randomly dispersed along dendritic segments, and their apparent avoidance of existing stable spines under control conditions is consistent with the idea that neighbouring spines share and compete for local resources18,19,20,21.
a, Schematic illustrating the measurement of Dn–s. b, The median of measured values of Dn–s (red circle) is significantly larger than that of simulated values of Dn–s (box plot of results from 1,000 simulations, with whiskers representing the minimum and the maximum) in control mice. The simulation is based on the null hypothesis that new spines are added independently and uniformly along a linear dendrite. c, Cumulative probability distribution of measured Dn–s is shifted towards longer distances than the simulated Dn–s in control mice. d, Schematic illustrating the measurement of Dn2–s, clustered. The nearest spine to a clustered n2 could be either a persistent first new spine (n1) or a stable spine existing since day 0, depending on relative n2 location. e, Dn1–s in control mice is comparable to that of trained mice. In trained mice, Dn2–s, clustered is significantly smaller than Dn1–s, whereas Dn2–s, non-clustered is comparable to Dn1–s. The number of spines analysed in each condition is indicated on each column. *P < 0.05. Error bars, s.e.m.
To determine if motor learning alters the spatial distribution of new spines, we examined values of Dn–s in mice trained with the reaching task. We found that the distance between a new spine formed on training day 1 (n1) and the nearest existing spine (Dn1–s) was comparable for trained and control mice (P > 0.7). We classified new spines formed between training days 1 and 4 (n2) into two categories: clustered n2 (that is, those that formed next to a stabilized first new spine; Fig. 3d) and non-clustered n2 (those that did not form next to a stabilized first new spine). We found that clustered n2 were significantly closer to their nearest stable spines (n1 or a stable spine existing since day 0; Fig. 3d) (Dn2–s, clustered) than were n1 (Dn1–s) (P < 0.05). In contrast, the distance between a non-clustered n2 and its nearest stable spine (Dn2–s, non-clustered) was comparable to Dn1–s (P > 0.9, Fig. 3e). In addition, when an n1 formed between two adjacent stable spines, the distance between the two stable spines (Ds–n1–s) was comparable for control and trained mice (P > 0.7; Supplementary Fig. 7). However, the distance between a stabilized n1 and the adjacent stable spine, between which a clustered n2 formed (Dn1–n2–s), was significantly smaller than the distance between two adjacent stable spines, between which a non-clustered n2 formed (Ds–n2–s; P < 0.01; Supplementary Fig. 7). These results suggest that learning-induced clustered new spines can overcome the spatial constraint of existing spines and be packed into tighter dendritic space.
Recent studies have shown that dendritic spines are dynamic in the living brain, and that rearrangement of cortical connections through de novo growth and loss of spines provides a structural substrate for experience-dependent plasticity22,23,24,25. Built upon these works, our study reveals a novel spatial rule of spinogenesis during motor learning. We found that learning-induced new spines tend to form in small clusters (mostly pairs). The correlation between the emergence of the second new spine and the strengthening of the first new spine also suggests their potential participation in the same neuronal circuit. These findings support the clustered plasticity model, which postulates that synapses located close together along the same dendritic branch are more likely to be allocated for the same information than synapses dispersed throughout the dendritic arbor7. Indeed, in the mouse auditory cortex, although spines tuned for different frequencies are highly interspersed, 26% of neighbouring spines exhibit similar effective frequencies, much more frequently than anticipated from random distribution (10%)26. Therefore, although neurons tend to maximize their overall connections27, clustered plasticity ensures strengthening of circuit-specific connections and enables spatial coding for task-related information.
Previous electron microscopy studies have revealed that neighbouring spines can form synapses with the same axon28,29,30 (see Supplementary Fig. 8a, c, e). Positioning multiple synapses between a pair of neurons in close proximity allows nonlinear summation of synaptic strength, and potentially increases the dynamic range of synaptic transmission well beyond what can be achieved by random positioning of the same number of synapses. Alternatively, clustered new spines may synapse with distinct (but presumably functionally related) presynaptic partners (Supplementary Fig. 8b, d). In this case, they could potentially integrate inputs from different neurons nonlinearly and increase the circuit’s computational power. Distinguishing between these two possibilities would probably require circuit reconstruction by electron microscopy following in vivo imaging to reveal the identities of presynaptic partners of newly formed spines.
Profiling spine formation during novel experiences, our data revealed a critical role of repetitive activation of the same neuronal circuit. The fact that the second new spine in a cluster can overcome the spatial constraint imposed by existing spines suggests that repetitive activation of a neuronal circuit may modify or reallocate local ‘resources’ for spinogenesis. Such resources may be permissive or instructive molecular cues at the pre- or postsynaptic site, or the availability of suitable partners (for example, axonal boutons). Understanding the nature and regulation of such resources may hold the key to elucidating the cellular mechanisms of clustered spine formation. It will be conducive to the development of tools to label and manipulate specific synaptic populations, and ultimately to the dissection of the causal relationship between synaptic dynamics and learning.
Methods Summary
YFP-H line mice14 expressing yellow fluorescent protein (YFP) in a small subset of cortical neurons were used in all the experiments. Mice of both sexes were trained with different motor-skill tasks or housed in a motor-enriched environment, starting at 1 month of age (see Methods). The procedures for transcranial two-photon imaging and quantification of spine dynamics have been described previously11,15. ImageJ was used to measure spine head size, as well as inter-spine distances. Simulation was performed with custom-written codes in Matlab (MathWorks) and statistical analyses were performed using GraphPad Prism 5 (GraphPad Software) (see Methods). All data were presented as mean ± s.e.m. P values were calculated using the Mann–Whitney U-test for independent samples, and the Wilcoxon signed-rank test for paired samples.
Online Methods
Experimental animals
Thy1-YFP-H line mice14 were obtained from the Jackson Laboratory. Mice were group-housed and bred in the University of California, Santa Cruz, animal facility, with all experiments performed in accordance with approved animal protocols.
Motor skill training and motor enrichment
Both the mouse single-seed reaching task and capellini-handling task protocols have been previously described15. ‘Motor-enriched’ mice were reared in groups of 8–12 in large cages (90 cm × 25 cm × 15 cm) containing various toys, such as ropes, ladders, chains, hanging mesh/bars etc., all of which required substantial motor coordination. The nature of toys was changed on a daily basis. Control mice were housed in standard mouse cages, with up to five mice per cage.
Surgical procedure for in vivo transcranial imaging
The procedure for transcranial two-photon imaging has been described previously11,31. Trained mice were imaged immediately after each training session.
Data quantification
All analyses of spine dynamics were done using ImageJ software, blinded for experimental conditions. Quantification criteria of dendritic spines have been described previously15. All dendritic protrusions were tracked manually in three-dimensional stacks to ensure the consistency of protrusion identification across imaging sessions, despite possible tissue movement or rotation. The number and location of dendritic protrusions (defined as protrusion length larger than one-third of dendritic shaft diameter) were identified in each view. Filopodia were identified as long, thin structures with the ratio of head diameter to neck diameter being less than 1.2 and the ratio of length to neck diameter being greater than 3. The remaining protrusions were classified as spines. Formation and elimination of spines and filopodia were determined by comparing images collected at two different time points. Spines or filopodia were considered identical between the two images if they were within 0.7 µm of their expected positions, based on their spatial relationship to adjacent landmarks and/or their positions relative to immediately adjacent spines. A stable spine was defined as a spine that was present in both images. A new spine was a spine that appeared in a subsequent image but was absent from the initial image. Percentages of formed and eliminated spines (or dendritic protrusions) were normalized to the number of spines (or dendritic protrusions) in the initial image. Spine diameter analyses have been previously described11. Because imaging and animal conditions varied over time, the ratio of the spine head diameter to the adjacent dendritic shaft diameter was used as the normalized spine head diameter. Measurement of spine head intensity, as described previously32, was also performed to confirm these spine size results. Briefly, we determined the signal intensity (defined as the sum intensity of all pixels composing the spine in the best focal plane) and subtracted the background intensity (defined as the sum intensity of a region composed of the same number of pixels as the spine but with no YFP-labelled structure). The difference was then divided by the mean intensity of the adjacent dendritic shaft (defined similarly as the difference between the mean signal intensity of the shaft and the mean background intensity) to correct for varying imaging conditions. The final value was termed ‘integrated spine brightness.’ All distance measurements were done in ImageJ. To simulate spine formation, we first obtained the relative location of stable spines by measuring inter-spine distances along traced dendrites in seven control animals, and concatenated dendritic segments from each animal into a single ‘synthetic dendrite.’ We then used custom-written Matlab codes to simulate the addition of new spines. As we observed, two spines can extend from the same linear location along the dendritic segment and point towards different directions, given the cylindrical shape of dendrites. In our analysis and simulation, we made the simplifying approximation that the dendritic segment is one-dimensional rather than a tube. Therefore, zero inter-spine distance in our analysis represents two spines overlapping in linear position but actually located at different sites around the circumference of the dendritic segment. In each round of simulation, the same numbers of new spines as observed in experiments were generated independently and uniformly along synthetic dendrites. The distance between each new spine and its nearest stable spine (Dn–s) was calculated. The simulation was repeated 1,000 times and the resultant data were pooled to compute the simulated sample median and the cumulative probability curve. All data were presented as mean ± standard error of mean (s.e.m.). P values were calculated using the Mann–Whitney U-test for independent samples, and the Wilcoxon signed-rank test for paired samples.
References
Silva, A. J., Zhou, Y., Rogerson, T., Shobe, J. & Balaji, J. Molecular and cellular approaches to memory allocation in neural circuits. Science 326, 391–395 (2009)
Aimone, J. B., Wiles, J. & Gage, F. H. Potential role for adult neurogenesis in the encoding of time in new memories. Nature Neurosci. 9, 723–727 (2006)
Han, J. H. et al. Selective erasure of a fear memory. Science 323, 1492–1496 (2009)
Komiyama, T. et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464, 1182–1186 (2010)
Kee, N., Teixeira, C. M., Wang, A. H. & Frankland, P. W. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nature Neurosci. 10, 355–362 (2007)
Larkum, M. E. & Nevian, T. Synaptic clustering by dendritic signalling mechanisms. Curr. Opin. Neurobiol. 18, 321–331 (2008)
Govindarajan, A., Kelleher, R. J. & Tonegawa, S. A clustered plasticity model of long-term memory engrams. Nature Rev. Neurosci. 7, 575–583 (2006)
Poirazi, P. & Mel, B. W. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron 29, 779–796 (2001)
Sjostrom, P. J. & Hausser, M. A cooperative switch determines the sign of synaptic plasticity in distal dendrites of neocortical pyramidal neurons. Neuron 51, 227–238 (2006)
Losonczy, A. & Magee, J. C. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron 50, 291–307 (2006)
Zuo, Y., Lin, A., Chang, P. & Gan, W. B. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron 46, 181–189 (2005)
Segal, M. Dendritic spines and long-term plasticity. Nature Rev. Neurosci. 6, 277–284 (2005)
Harms, K. J. & Dunaevsky, A. Dendritic spine plasticity: looking beyond development. Brain Res. 1184, 65–71 (2007)
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000)
Xu, T. et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919 (2009)
Harvey, C. D. & Svoboda, K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature 450, 1195–1200 (2007)
De Roo, M., Klauser, P. & Muller, D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 6, e219 (2008)
Gray, N. W., Weimer, R. M., Bureau, I. & Svoboda, K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo. PLoS Biol. 4, e370 (2006)
Harvey, C. D., Yasuda, R., Zhong, H. & Svoboda, K. The spread of Ras activity triggered by activation of a single dendritic spine. Science 321, 136–140 (2008)
Rose, J., Jin, S. X. & Craig, A. M. Heterosynaptic molecular dynamics: locally induced propagating synaptic accumulation of CaM kinase II. Neuron 61, 351–358 (2009)
Tsuriel, S. et al. Local sharing as a predominant determinant of synaptic matrix molecular dynamics. PLoS Biol. 4, e271 (2006)
Yu, X. & Zuo, Y. Spine plasticity in the motor cortex. Curr. Opin. Neurobiol. 21, 169–174 (2011)
Chen, J. L. & Nedivi, E. Neuronal structural remodeling: is it all about access? Curr. Opin. Neurobiol. 20, 557–562 (2010)
Fu, M. & Zuo, Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 34, 177–187 (2011)
Holtmaat, A. & Svoboda, K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature Rev. Neurosci. 10, 647–658 (2009)
Chen, X., Leischner, U., Rochefort, N. L., Nelken, I. & Konnerth, A. Functional mapping of single spines in cortical neurons in vivo. Nature 475, 501–505 (2011)
Yuste, R. Dendritic spines and distributed circuits. Neuron 71, 772–781 (2011)
Knott, G. W., Holtmaat, A., Wilbrecht, L., Welker, E. & Svoboda, K. Spine growth precedes synapse formation in the adult neocortex in vivo. Nature Neurosci. 9, 1117–1124 (2006)
Fiala, J. C., Allwardt, B. & Harris, K. M. Dendritic spines do not split during hippocampal LTP or maturation. Nature Neurosci. 5, 297–298 (2002)
Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R. & Muller, D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402, 421–425 (1999)
Grutzendler, J., Kasthuri, N. & Gan, W. B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002)
Hofer, S. B., Mrsic-Flogel, T. D., Bonhoeffer, T. & Hubener, M. Experience leaves a lasting structural trace in cortical circuits. Nature 457, 313–317 (2009)
Acknowledgements
We thank D. States, D. Garcia, L. Hinck, T. Jones, S. Song, W. Thompson and G. Wang for comments on this manuscript. We thank A. Perlik and T. Xu for technical support. This work was supported by grants from the DANA Foundation and the National Institutes of Mental Health to Y.Z.
Author information
Authors and Affiliations
Contributions
M.F. and X.Y. did the in vivo imaging and made the figures. M.F. performed behavioural training and all spine analyses, and made figures for repetitive imaging. J.L. and M.F. performed Matlab simulation and statistical analyses. J.L., M.F. and X.Y. participated in discussion about the paper. Y.Z. initiated and designed the project, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Notes 1-2, additional references, Supplementary Figures 1-8 with legends and Supplementary Table 1. (PDF 980 kb)
Rights and permissions
About this article
Cite this article
Fu, M., Yu, X., Lu, J. et al. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 483, 92–95 (2012). https://doi.org/10.1038/nature10844
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10844
This article is cited by
-
Engram mechanisms of memory linking and identity
Nature Reviews Neuroscience (2024)
-
Real-time visualization of structural dynamics of synapses in live cells in vivo
Nature Methods (2024)
-
VAP spatially stabilizes dendritic mitochondria to locally support synaptic plasticity
Nature Communications (2024)
-
Cell-specific IL-1R1 regulates the regional heterogeneity of microglial displacement of GABAergic synapses and motor learning ability
Cellular and Molecular Life Sciences (2024)
-
Task-specific modulation of corticospinal neuron activity during motor learning in mice
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.