Abstract
Myeloid cells are a feature of most tissues. Here we show that during development, retinal myeloid cells (RMCs) produce Wnt ligands to regulate blood vessel branching. In the mouse retina, where angiogenesis occurs postnatally1, somatic deletion in RMCs of the Wnt ligand transporter Wntless2,3 results in increased angiogenesis in the deeper layers. We also show that mutation of Wnt5a and Wnt11 results in increased angiogenesis and that these ligands elicit RMC responses via a non-canonical Wnt pathway. Using cultured myeloid-like cells and RMC somatic deletion of Flt1, we show that an effector of Wnt-dependent suppression of angiogenesis by RMCs is Flt1, a naturally occurring inhibitor of vascular endothelial growth factor (VEGF)4,5,6. These findings indicate that resident myeloid cells can use a non-canonical, Wnt–Flt1 pathway to suppress angiogenic branching.
Similar content being viewed by others
Main
Myeloid cells have a wide array of biological activities that include immune activation, arteriogenesis7, and regulation of salt balance and blood pressure8. Myeloid cells also regulate vascularity. Tumour-associated macrophages influence the growth of blood vessels9 in part because they are a source of VEGFA10. Myeloid cells also promote angiogenic branching11 and anastamosis12. Depending on the context, myeloid cells can be either anti-angiogenic13 or pro-angiogenic14. Here we show that RMCs suppress retinal angiogenesis via a Wnt–Flt1 pathway (Supplementary Fig. 1).
Retinal angiogenesis begins on the day of birth in the mouse with the formation of a superficial vascular plexus (Fig. 1a) that lies within the ganglion cell layer15. After formation of this superficial plexus by post-partum day 7 (P7), angiogenic sprouts descend vertically through the retinal layers from P8 to P14 (Fig. 1a). At the outer edge of the inner nuclear layer (INL) the vertical angiogenic sprouts turn and simultaneously branch to form a deep vascular plexus (Fig. 1a). Using antibodies to the vascular endothelial cell (VEC) marker endomucin and to the green fluorescent protein (GFP) of the c-fms–EGFP (also known as Tg(Csf1r-EGFP)1Hume) transgene that marks RMCs, we show that myeloid cells have a unique spatial relationship with angiogenic tip cells. At the point of turning and branching in the outer INL, myeloid cells and angiogenic sprouts are in close contact (Supplementary Fig. 2a). This was confirmed by labelling with isolectin and F4/80 (F4/80 is also known as Emr1; Supplementary Fig. 2b). We then took advantage of high-intensity isolectin labelling of VECs and RMCs and performed a three-dimensional reconstruction with false colouring to illustrate the overall topology of the angiogenic tip cell–RMC interaction (Fig. 1b). This showed close contact between the two cell types throughout turn-and-branch angiogenesis in the deep retinal layer. Furthermore, after turning, angiogenic tip cells extend within the plane of the deep retinal layer and remain RMC-associated (Fig. 1c). In further defining RMCs, we showed that CD204 (also known as Msr1) was unique to RMCs with amoeboid morphology in the superficial retinal layer (Supplementary Fig. 2c, d). By contrast, deep retinal layer RMCs with extended morphology were CD204-negative (Supplementary Fig. 2c, e). Both layers of RMCs expressed Iba1 (ref. 16) but at different levels (Supplementary Fig. 2c–e). This information allowed us to sort distinct populations of superficial (CD11b+, F4/80+, CD204+) and deep layer (CD11b+, F4/80+, CD204-) RMCs by flow cytometry (Fig. 1d, e).
a, Schematic of retina at postnatal days (P) 2, 6, 10, and 18. RMCs interacting with descending vertical sprouts are labelled with red arrowheads. Adapted from ref. 1. b, Isolectin-labelled three-dimensional reconstruction of vertical angiogenic sprouts (green) and RMCs (false-colour red). c, As in b but a two-dimensional image in the deep vascular layer. Scale bars, 5 μm. d, e, Flow cytometry of deep layer RMCs based on surface markers. 7-AAD, 7-aminoactinomycin D. f, PCR for Wnt pathway components on flow-sorted RMCs. Red arrowheads indicate expected sizes. Act, β-actin.
On the basis of previous work showing vascular regulation by myeloid Wnt ligands17 we proposed that RMCs might use Wnt ligands to regulate retinal angiogenesis. First, we examined the expression of Wnt ligands and receptors in superficial and deep RMCs isolated by flow cytometry. PCR with reverse transcription (RT–PCR) analysis of the deep RMC population showed expression of Wnt5a, Wnt6 and Wnt11, Fzd7 and Fzd8 as well as the co-receptor Lrp5 (Fig. 1f). With the exception of Wnt5b, which was inconsistent, no other Wnt ligand was detected in deep RMCs. Superficial RMCs expressed similar Wnt and Fzd proteins (Fig. 1f), but also expressed Wnt2b, 3 and 3a (data not shown).
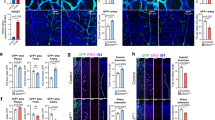
The challenge of genetic analysis when RMCs express many Wnt ligands was addressed by the generation of a loxP-flanked conditional allele for the essential Wnt ligand transporter Wls2,3. Both superficial and deep RMCs expressed Wls (Fig. 1f). Wls was deleted using the myeloid cre driver cfms–icre18, which we confirmed was functional in RMCs (Supplementary Fig. 3a–c). To analyse retinal vasculature, we imaged superficial and deep retinal vessels at P18 (Fig. 2a). Quantification of vessel branch points showed that cfms–icre alone had no effect (Fig. 3b). Furthermore, compared with control Wlsfl/+ mice, Wlsfl/+; cfms–icre animals had a normal superficial vascular plexus (Fig. 2a c). By contrast, the deep vascular layer (Fig. 2a, c) showed an overgrowth. Interestingly, no further enhancement of vascular overgrowth was apparent when the myeloid Wls deletion was homozygous as in Wls−/fl; cfms–icre mice (data not shown). Because myeloid cells are positioned below descending vessels at P10, we assessed vessel branching at the base of these sprouts. Wlsfl/+; cfms–icre mice showed reduced simple turning (no branches) and single branching, but significantly more multi-branch events (Fig. 2d). As a weighted mean, the branch index was 2.0 in control and 3.2 in the mutant (P < 0.0001).
a, Isolectin labelling of superficial and deep retinal vasculature in Wlsfl/+ and Wlsfl/+; cfms–icre mice. Scale bars, 50 μm. b, c, P18 vessel branch points in labelled genotypes. n = 4 (b), n = 8 (c). d, Branches emanating from the base of vertical sprouts in the P10 deep vascular layer. n = 8. b–d used Student’s t-test (two-tailed). e, Time-course of deep layer branches in indicated genotypes. Shading shows when angiogenesis (green) and remodelling (red) predominate. One-way ANOVA showed P = 0.0021. n = 4 for each point. Error bars are s.e.m., NS, not significant.
a, b, Intracellular Ca2+ in RAW264.7 cells treated with Wnt5a (a) or supernatant from Wlsfl/fl MEFs expressing Wnt5a or Wnt5a and cre (b). One Way ANOVA showed P ≤ 0.0001 for both. c, d, Isolectin labelling of superficial and deep retinal vasculature in Lrp5fl/+ (c) and Lrpfl/fl; cfms–icre (d) mice. 50 μm scale bars. e, P18 vessel branch points in labelled genotypes. f, Vertical vessels connecting to the deep layer in labelled genotypes. e, f, Used one-way ANOVA with Tukey’s post-hoc. n ≥ 8 per genotype. Errors are s.e.m., NS, not significant.
The higher vascular density of the deep layer in Wlsfl/+; cfms–icre mice could reflect enhanced angiogenesis or defective remodelling. To assess this, we counted deep layer branch points in control (Wlsfl/+ ) and experimental (Wlsfl/+; cfms–icre) mice over a time-course from P6 to P18 (Fig. 2e). Between P14 and P18 when remodelling predominates (Fig. 2e, red zone) control and mutant graph slopes were nearly identical. By contrast, between P6 and P14 when vessel growth predominates (Fig. 2e, green zone), the slope of the graph is greater in the mutant. There was no difference between control and mutant animals in the number of vertical sprouts connecting superficial and deep layers (Supplementary Fig. 3d). Combined, the data in Fig. 2 are consistent with a model in which myeloid Wnt ligands suppress branch formation as angiogenic sprouts make contact with deep layer RMCs (Fig. 1b, c). These data were corroborated by the vascular overgrowth phenotype of Wnt5a (ref. 19) and Wnt11 (ref. 20) heterozygotes (Supplementary Figs 3e, f and 4).
Wnt5a and Wnt11 are most often associated with non-canonical Wnt signalling21. We thus investigated the possibility that Wnt-dependent suppression of deep retinal angiogenesis was a non-canonical response. Wnt3a, but not Wnt5a, elicited a canonical response in SuperTopflash (STF) reporter cells (Supplementary Fig. 5a). Some non-canonical Wnt pathways activate a Ca2+ flux21. To determine whether myeloid Wnt proteins elicited a Ca2+ flux, we added Wnt5a to myeloid-like RAW264.7 cells and measured Ca2+-dependent Fura-2 dye emission. Recombinant Wnt5a increased intracellular Ca2+ compared to untreated controls (Fig. 3a and Supplementary Fig. 5b, c). Furthermore, to assess the requirement for Wls in Wnt ligand secretion, we exposed RAW264.7 cells to medium from Wlsfl/fl mouse embryonic fibroblasts (MEFs) transfected with Wnt5a or Wnt5a and cre recombinase plasmids. RAW264.7 cells had increased Ca2+ flux in response to Wnt5a-transfected MEF medium relative to Wnt5a- and cre-transfected medium (Fig. 3b). This validates the role of Wls in secretion of Wnt5a. Combined, these data show that one myeloid Wnt, Wnt5a, does not stimulate a canonical Wnt response, but can elicit a Ca2+ flux characteristic of some non-canonical Wnt responses.
In recent work, it has been shown that Wnt5a loss-of-function can rescue the defects associated with deletion of the canonical Wnt pathway co-receptors Lrp5 and Lrp6 (ref. 22). This finding has suggested that Lrp5/6 deletion actually represents a non-canonical pathway gain-of-function and that in deleting the non-canonical ligand Wnt5a, there is a re-balancing of pathway activity. This hypothesis is supported by biochemical analysis showing that Wnt5a can bind Lrp6, but does not elicit the phosphorylation required for canonical signalling22. Canonical, non-canonical reciprocal pathway inhibition has also been demonstrated23. These findings argue that a deletion of Lrp5/6 can define whether a Wnt signalling pathway is canonical or non-canonical. If it is canonical, an Lrp5/6 deletion will give the same phenotype as ligand deletion. By contrast, if the signalling pathway is non-canonical, the consequence of Lrp5/6 deletion would be opposite to that of ligand mutation. Thus, to determine whether the RMC response was canonical or non-canonical, we generated a cfms–icre somatic mutant of the Lrp5 coreceptor that is expressed in RMCs (Fig. 1f). Because the result was significantly diminished deep vascular layer density in somatic homozygotes (Fig. 3c–f), a response opposite to ligand deletion, this provides in vivo evidence that the Wnt response is non-canonical. Even though the superficial vascular layer was slightly deficient in somatic homozygotes (Fig. 3e) this was not the reason for reduced density in the deep vascular layer as the descending sprout number was unchanged (Fig. 3f).
The VEGF receptor Flt1 (also known as VEGFR1) can suppress angiogenesis because it has limited signalling capacity, a higher affinity for VEGF than Flk1 (also known as VEGFR2) and can sequester VEGF5,6. Alternative splicing produces both membrane-tethered and soluble forms5. Flt1 is known to contribute to corneal avascularity4 as well as the selection of angiogenic tip cells when expressed regionally in an existing vessel24. Flt1 is also known to be expressed in some myeloid populations6. Because angiogenesis in the deep retinal layers is VEGF-dependent25, Flt1 was a good candidate to mediate Wnt-dependent angiogenic suppression by deep RMCs.
We thus determined whether Flt1 was expressed in P12 RMCs by performing end-point RT–PCR on flow-sorted cells using primers that detected both transcripts. This showed that deep RMCs expressed Flt1 (Fig. 4a). By contrast, superficial RMCs did not (Fig. 4a). We also showed that Iba1-labelled RMCs positioned at the outer edge of the INL labelled with anti Flt1 antibodies (Fig. 4b). We then generated an RMC Flt1 loss-of-function using cfms–icre and the Flt1fl conditional allele26. Quantification of retinal vascular branch points in Flt1fl/+ ; cfms–icre mice at P18 showed that the superficial layer was unaffected but that the deep layer showed an increase in density (Fig. 4c, d). As with Wls somatic mutants, conditional homozygosity for Flt1 (Flt1fl/fl ; cfms–icre) did not give a further significant increase in vascular density (data not shown). Furthermore, the number of deep RMCs was unchanged (Supplementary Fig. 2g).
a, PCR for Flt1 transcript in flow-sorted RMCs. β-act, β-actin transcript. b, P14 retinal sections labelled for Flt1 and Iba1. Scale bars, 25 μm. c, P18 vessel branch points in the superficial and deep vasculature in Flt1fl/+ and Flt1fl/+; cfms–icre mice (n = 7, Student’s t-test). n = 7. d, Isolectin labelling of the retinal vasculature in labelled genotypes. Scale bars, 50 μm. e, PCR for indicated transcripts in E11.5 whole embryo (con) and RAW264.7 cells. f, g, quantitative PCR (f) for soluble Flt1 and membrane-tethered Flt1 in Wnt-treated RAW264.7 cells and ELISA (g) for soluble Flt1 on medium from Wnt-treated RAW264.7 cells (n = 4, one-way ANOVA). h, Quantitative PCR for soluble Flt1 in flow-sorted deep RMCs from Wlsfl/+ and Wlsfl/+; cfms–icre mice (n = 4, Student’s t-test). Errors are s.e.m.; NS, not significant.
These data showed that conditional deletion of Flt1 produced an enhancement of angiogenesis similar to that observed with conditional deletion of Wls. One implication was that Wls and Flt1 might function in the same angiogenesis suppression pathway. To test the possibility that Flt1 might be regulated by Wnt ligands, we used myeloid-like RAW264.7 cells that, like RMCs, express Fzd7 and Fzd8 (Fig. 4e). When stimulated with the ‘canonical’ ligand Wnt3a, RAW264.7 did not change the level of Flt1 transcript according to quantitative PCR (Fig. 4f). However, stimulation with Wnt5a produced a threefold increase in the level of both Flt1 isoform transcripts (Fig. 4f). Furthermore, an enzyme-linked immunosorbent assay (ELISA) showed that there was an increased level of soluble Flt1 in conditioned media from Wnt5a-stimulated RAW264.7 cells (Fig. 4g). Wnt3a stimulation again had no effect (Fig. 4g).
As a stringent test of the possibility that Wnt ligands stimulated expression of Flt1 in deep RMCs, we flow-sorted the CD11b+, F4/80+, CD204− population from both control (Wlsfl/+ ) and experimental (Wlsfl/+; cfms–icre) mice at P12 and performed quantitative PCR for Flt1 transcripts. We were never able to amplify the membrane-tethered Flt1 transcript but showed that there was a 90% reduction in transcript level for soluble Flt1 (Fig. 4h). This was consistent with the maximal phenotype observed in the heterozygous conditional Wls mutants. Although a heterozygous phenotype is not unusual, it is perhaps surprising that conditional homozygosity gave no further significant change. This kind of response might be explained by a low signalling sensitivity threshold for the Wnt–Flt1 pathway.
The data we describe show that RMCs can modulate angiogenic responses directly by producing the VEGF inhibitory receptor Flt1. Unexpectedly, we also show that the production of Flt1 depends on myeloid non-canonical Wnt ligands. In this setting, myeloid Wnt ligands might function in autocrine stimulation as has been documented for cultured macrophages27 or might operate via a more complex pathway involving another cell type as an intermediate. The Wnt–Flt1 response represents a new pathway for the regulation of VEGF-stimulated angiogenesis. The targeting of appropriate Fzd receptors or other Wnt–Flt1 pathway components may offer new opportunities to modulate the production of Flt1 and thus, the VEGF-stimulated angiogenic response. Because macrophage-related cells are ubiquitous and highly mobile, it is possible that the Wnt–Flt1 pathway will be a general means to suppress VEGF locally and thus to restrain vascular responses. In future studies it will be interesting to determine, for example, if this pathway is active in the suppression of wound angiogenesis by macrophages13 or is inactivated in macrophage-dependent tumour angiogenesis28.
Additionally, our observations are consistent with a string of recent papers describing the role of myeloid cells beyond their well-documented functions in innate immunity7,8,17,29. These findings build on an idea articulated nearly a century ago30. It was suggested then that phagocytes originally evolved to regulate developmental processes, and that their immune functions were a later evolutionary adaptation. Macrophages were proposed to be the “policemen” of multi-cellular organisms, and that they could establish “harmony from chaos”. Here we show, in the setting of the retina, that myeloid cells do just that by fine-tuning vascular density and directing vascular traffic.
Methods Summary
We prepared and stained retinas as reported previously1. To isolate RMCs, we digested retinas, pre-enriched with CD11b beads, and sorted for surface makers with the FACSAria II. To obtain conditioned medium from MEFs, we performed transient transfections with Wnt5a, Thy1.1 and cre plasmids, sorted Thy1.1-positive MEFS, and re-plated transfected cells. We performed calcium imaging on RAW264.7 cells loaded in Ringer’s solution with 5 μM Fura-2 AM and imaged at 510 nm at 1 Hz after excitation at 340 nm and 380 nm. All animal experiments were performed in accordance with IACUC-approved guidelines and regulations.
Online Methods
Quantification of retinal vasculature and isolation of RMCs
Retinas were prepared and imaged as reported1 except that Alexa Fluor 488 isolectin GS-IB4 (Invitrogen) was used to label retinal vessels and RMCs. Other antibodies included anti-endomucin (Santa Cruz, V.7C7), anti-GFP (Abcam), anti-Iba1 (Wako), anti-CD204 (AbD Serotec), and anti-Flt1 (R&D). For quantification, we used 200× magnification images located at the retinal periphery between artery and vein. For each genotype, at least three fields were analysed from at least four animals from a minimum of two litters. Control and experimental animals were littermates. This minimized the effect of strain background in producing variation in vascular density31,32,33,34. For flow sorting, retinas were digested and sorted as reported16 except that 0.5 mg ml−1 DNase II (Sigma Aldrich) was used. Furthermore, myeloid cells were pre-enriched using CD11b beads (Miltenyi Biotech). Cells were then incubated with anti-CD16/32 (clone 24.G2) for 30 min and labelled with monoclonal antibodies to phycoerythrin-Cy7 conjugated anti-CD11b (clone M1/70), allophycocyanin-Cy7 or peridinin chlorophyll A protein-Cy5.5 conjugated anti-F4/80 (clone BM8), Alexa-647 conjugated anti-CD204 (clone 2F8), and 7-aminoactinomycin D. Cells were sorted with a FACSAria II running DiVa software.
RNA isolation and RT–PCR
RNA was isolated using RNeasy (Qiagen). Quantitative PCR was performed with QuantiTect SYBR green (Qiagen). Primers are listed in Supplementary Table 1.
In vitro analysis
MEFs were isolated as described35 from Wlsfllfl mice36 and transfected with combinations of 2.5 μg Wnt5a, Thy1.1 and cre plasmids using TransIT-2020 (Mirus). MEFs were sorted using magnetic beads for Thy1.1, replated and supernatant was collected after 24 h. RAW264.7 cells (ATCC) were grown in DMEM (10% FBS) and treated with recombinant Wnt3a (10 ng ml−1, R&D) or Wnt5a (500 ng ml−1, R&D). ELISA was performed using the sVEGFR1 Quantikine kit (R&D). For calcium imaging, RAW264.7 cells were loaded in Riger’s solution with 5 μM Fura-2 AM and imaged at 510 nm at 1 Hz after excitation at 340 nm and 380 nm. Data was acquired using EasyRatioPro. Ca2+ traces are 50-cell averages from two experiments.
Statistics
All statistical tests used are stated in the figure legends. In analysing quantitative PCR data, the P values refer to a comparison of the ΔΔCt values.
Animals
Breeding and genotyping of Wlsfl (ref. 36), cfms–icre (ref. 18), Z/EG (ref. 37), Wnt5a+/− (ref. 19), Wnt11+/− (ref. 20) and Fltfl (ref. 26) was performed as previously described. Lrp5fl will be described in detail in a forthcoming publication. The allele is a conventional design where exon 2 of the Lrp5 gene is flanked by LoxP sites. It has been confirmed that deletion between the LoxP sites produces a loss-of-function. All animal experimentation was carried out using protocols approved by the Institutional Animal Care and Use Committee.
References
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 (2003)
Ching, W. & Nusse, R. A dedicated Wnt secretion factor. Cell 125, 432–433 (2006)
Carpenter, A. C., Rao, S., Wells, J. M., Campbell, K. & Lang, R. A. Generation of mice with a conditional null alelle for Wntless . Genesis 48, 554–558 (2010)
Ambati, B. K. et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 443, 993–997 (2006)
Kendall, R. L. & Thomas, K. A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl Acad. Sci. USA 90, 10705–10709 (1993)
Shibuya, M. Structure and dual function of vascular endothelial growth factor receptor-1 (Flt-1). Int. J. Biochem. Cell Biol. 33, 409–420 (2001)
Pipp, F. et al. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ. Res. 92, 378–385 (2003)
Machnik, A. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nature Med. 15, 545–552 (2009)
Lin, E. Y. & Pollard, J. W. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 67, 5064–5066 (2007)
Stockmann, C. et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456, 814–818 (2008)
Kubota, Y. et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J. Exp. Med. 206, 1089–1102 (2009)
Fantin, A. et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116, 829–840 (2010)
Martin, P. et al. Wound healing in the PU.1 null mouse–tissue repair is not dependent on inflammatory cells. Curr. Biol. 13, 1122–1128 (2003)
Grunewald, M. et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124, 175–189 (2006)
Saint-Geniez, M. & D'Amore, P. A. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int. J. Dev. Biol. 48, 1045–1058 (2004)
Mendes-Jorge, L. et al. Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood-retinal barrier. Invest. Ophthalmol. Vis. Sci. 50, 5997–6005 (2009)
Lobov, I. B. et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature 437, 417–421 (2005)
Deng, L. et al. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am. J. Pathol. 176, 952–967 (2010)
Yamaguchi, T. P., Bradley, A., McMahon, A. P. & Jones, S. A. Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126, 1211–1223 (1999)
Majumdar, A., Vainio, S., Kispert, A., McMahon, J. & McMahon, A. P. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130, 3175–3185 (2003)
Seifert, J. R. & Mlodzik, M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nature Rev. Genet. 8, 126–138 (2007)
Bryja, V. et al. The extracellular domain of Lrp5/6 inhibits noncanonical Wnt signaling in vivo . Mol. Biol. Cell 20, 924–936 (2009)
Grumolato, L. et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 24, 2517–2530 (2010)
Chappell, J. C., Taylor, S. M., Ferrara, N. & Bautch, V. L. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev. Cell 17, 377–386 (2009)
Haigh, J. J. et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev. Biol. 262, 225–241 (2003)
Lichtenberger, B. M. et al. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 140, 268–279 (2010)
Blumenthal, A. et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood 108, 965–973 (2006)
Stockmann, C. et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature 456, 814–818 (2008)
Lin, S. L. et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc. Natl Acad. Sci. USA 107, 4194–4199 (2010)
Tauber, A. I. Metchnikoff and the phagocytosis theory. Nature Rev. Mol. Cell Biol. 4, 897–901 (2003)
Rohan, R. M., Fernandez, A., Udagawa, T., Yuan, J. & D’Amato, R. J. Genetic heterogeneity of angiogenesis in mice. FASEB J. 14, 871–876 (2000)
Gao, G. et al. Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium–derived factor in Brown Norway and Sprague Dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes 51, 1218–1225 (2002)
Chan, C. K. et al. Mouse strain-dependent heterogeneity of resting limbal vasculature. Invest. Ophthalmol. Vis. Sci. 45, 441–447 (2004)
Chan, C. K. et al. Differential expression of pro- and antiangiogenic factors in mouse strain-dependent hypoxia-induced retinal neovascularization. Lab. Invest. 85, 721–733 (2005)
Nagy, A., Gertsenstein, M., Vintersten, K. & Behringer, R. Manipulating the mouse embryo: a laboratory manual. 3rd edn, 371–373 (Cold Spring Harbor Laboratory Press, 2003)
Carpenter, A. C., Rao, S., Wells, J. M., Campbell, K. & Lang, R. A. Generation of mice with a conditional null allele for Wntless . Genesis 48, 554–558 (2010)
Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C. G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147–155 (2000)
Acknowledgements
We thank P. Speeg for technical assistance and A. P. McMahon for the Wnt11 mice. This work was supported by the NIH (J.A.S., M.W-K., J.W.P., J.D.M., T.Y., B.O.W., R.A.L.) by the HHMI (J.D.M.) and Cancer Research UK (H.G.).
Author information
Authors and Affiliations
Contributions
R.A.L provided project leadership and wrote the manuscript with J.A.S. J.A.S., I.L., S.R., H.G., and R.A.L. designed the experiments. J.A.S, I.L., S.R., G.M., A.C.C., A.R.B., J.F., and R.A. performed the experiments. S.R., J.W.P., T.Y., N.F. and B.O.W developed critical reagents. Experimental supervision and helpful discussions were provided by M.W-K., J.D.M., S.R., J.W.P., and H.G.
Corresponding author
Ethics declarations
Competing interests
[Competing interest: N.F. is an employee of Genentech Inc. The authors declare no other potential conflicts of interest.]
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-5 with legends, Supplementary Table 1 and additional references. (PDF 3021 kb)
Rights and permissions
About this article
Cite this article
Stefater III, J., Lewkowich, I., Rao, S. et al. Regulation of angiogenesis by a non-canonical Wnt–Flt1 pathway in myeloid cells. Nature 474, 511–515 (2011). https://doi.org/10.1038/nature10085
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10085
This article is cited by
-
Amyloid-β (Aβ) immunotherapy induced microhemorrhages are associated with activated perivascular macrophages and peripheral monocyte recruitment in Alzheimer’s disease mice
Molecular Neurodegeneration (2023)
-
Exploiting synergistic effect of CO/NO gases for soft tissue transplantation using a hydrogel patch
Nature Communications (2023)
-
The role of macrophages in the tumor microenvironment and tumor metabolism
Seminars in Immunopathology (2023)
-
Non-canonical WNT signalling in cardiovascular disease: mechanisms and therapeutic implications
Nature Reviews Cardiology (2022)
-
Neural crest cell-derived pericytes act as pro-angiogenic cells in human neocortex development and gliomas
Fluids and Barriers of the CNS (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.