Abstract
The relationships among the living apes and modern humans have effectively been resolved, but it is much more difficult to locate fossil apes on the tree of life because shared skeletal morphology does not always mean shared recent evolutionary history. Sorting fossil taxa into those that belong on the branch of the tree of life that leads to modern humans from those that belong on other closely related branches is a considerable challenge.
Similar content being viewed by others
Main
The researchers who discovered and analysed the fossil evidence for Ardipithecus ramidus1 are emphatic that it is an early member of the hominin clade, and comparable claims have been made by other research groups for earlier fossil evidence2,3,4. This review examines the difficulties of substantiating these claims by exploring the evolutionary context of the earliest hominins. We offer alternative interpretations for where Ardipithecus, Orrorin and Sahelanthropus might be accommodated within the tree of life.
Relationships and ancestors
All the organisms alive today are the terminal twigs of the crown of the tree of life and all the organisms that have ever lived are on branches within the tree. Most branches end before they reach the crown and so, even though modern-day diversity is impressive, the animals alive today are only a tiny fraction of all the types of animals that have lived in the past. In the nineteenth century the only evidence that could be used to relate living taxa was the gross morphology of their hard and soft tissues. During the first half of the twentieth century new lines of molecular evidence began to emerge5,6,7, followed most recently by genomic evidence8,9,10. The conclusions from all of these lines of evidence are consistent with the results of a recent molecular supermatrix analysis based on both mitochondrial and nuclear genes11 that supports a (((Pan, Homo) Gorilla) Pongo) pattern of relationships among humans and great apes. This all points to chimpanzees and bonobos (that is, Pan) and their extinct close relatives (called panins) being more closely related to modern humans and our extinct close relatives (called hominins) than to gorillas (Table 1); therefore, hominins and panins are referred to as sister taxa. Some of these data can be used to generate hypotheses about the timing of the splitting events that are implied by these relationships. Several lines of evidence9,10,11 suggest that the common ancestor of panins and hominins probably lived in the period between 8 and 4 million years ago, and most probably between 6 and 4 million years ago.
Despite being each other’s closest living relatives, there are still substantial morphological, molecular and behavioural differences between living chimpanzees and bonobos on the one hand, and modern humans on the other. It is simplistic to assume that only hominins have undergone significant evolutionary change since the most recent common ancestor (MRCA) of panins and hominins: the African fossil record suggests that very few, if any, mammalian lineages have remained unchanged since the late Miocene (10 to 5 million years ago)12. However, there are sound logical reasons based on the morphology of their nearest modern outgroups to support the inference that the skeleton of the panin/hominin MRCA would have had more in common with chimpanzees and bonobos than with modern humans13.
Reconstructing ancestors
An important concept in phylogenetic reconstruction is the ancestral morphotype, a compilation of the derived features primitively shared by sister taxa such as Pan and Homo. The degree to which those taxa are more or less divergent from their last common ancestor affects the ease with which one can retrodict their shared ancestral morphology from the terminal states14. In cases where they are divergent, ancestral morphotypes can be imperfect or even misleading approximations with relatively low resolution, rather than precise and accurate formulations of the ancestral condition. Given that the extant apes and modern humans represent relict and probably highly specialized terminal members of what were once diverse radiations14, the accurate reconstruction of ancestral morphotypes among the hominoids has proved problematic.
Recent efforts to redefine the nested set of ancestral morphotypes of hominoids proceeding from the assumption that Ar. ramidus is a stem hominin1 nicely illustrate the inherent problems. Should the discovery of a purported fossil hominin overturn predictions about an ancestral morphotype based on a wealth of comparative data from extant taxa, or should one defer to the hypothetical morphotypes that best fit the comparative evidence and critically reassess the phylogenetic placement of fossil taxa that contradicts such an hypothesis? If Ardipithecus is in fact not a hominin then it would require (as noted by its supporters15) the confluence of a number of shared specializations developed in parallel between Ardipithecus and later hominins, but the opposite scenario, in which Ardipithecus is assumed to be a hominin, requires remarkably high levels of homoplasy among extant great apes1. These different appeals to Occam’s razor are predicated on the scale of the phylogenetic perspective. The former is a taxonomically more inclusive and hominoid-centric perspective; the latter hominin-centric perspective is taxonomically more exclusive.
On a related theme, when assessing the phylogenetic relationships of extinct hominids, comparisons are often limited to modern humans or great apes, but this simple dichotomy can lead to incorrect assumptions about interpretations of morphocline polarities and the functional–behavioural associations of particular features. The temptation is to assume that features that distinguish modern humans from great apes are related to the unique behaviours of modern humans. However, the Miocene ape precursors of extant great apes and modern humans were anatomically, and presumably behaviourally, quite different from modern great apes, and many of the features often associated uniquely with modern humans are likely to be primitive retentions or specializations that have broader functional–behavioural relationships. For example, the position and orientation of the foramen magnum and aspects of the anatomy of the pelvis and proximal femur that distinguish modern humans from great apes, typically identified as being uniquely related to bipedalism, are, in fact, also found in non-hominoid primates associated with quite different locomotor behaviours. To resolve this problem a broader comparative context is needed to be able to delineate the universe of functional–behavioural possibilities, as well as a more critical appreciation that modern human-like features absent among extant great apes should not simply be presumed to be autapomorphies (that is, unique specializations) of the hominin lineage. A sideways look at closely related extant and extinct taxa should be an important component of any analysis of a purported early hominin.
The detailed justifications for including Sahelanthropus, Orrorin and Ardipithecus in the hominin clade vary according to what anatomical regions are represented1,2,3,4, but three main threads run through the claims for hominin status. The first involves a reduction in size and a change in morphology of the canines accompanied by the partial or complete loss of upper canine/lower third premolar (P3) honing and a reduction in the degree of canine sexual dimorphism, associated with inferences about social organization. The second involves the location and orientation of the foramen magnum and inferences about upright posture, and the third involves features of the pelvis and other preserved postcranial elements that imply a dependence on bipedalism. In each case the assumption is that these character complexes and their inferred behaviours are unique and thus confined to the hominin clade.
The canine morphology that Ardipithecus and Sahelanthropus share with later hominins is, perhaps, the most convincing evidence to support their hominin status, but it is important to recognize that during the late Miocene a number of Eurasian hominids (for example, Oreopithecus, Ouranopithecus and Gigantopithecus) also developed small canines in conjunction with reduced canine–premolar honing, presumably as a result of parallel shifts in dietary behaviour in response to changing ecological conditions. Thus, these changes are in fact not unique to hominins and it is conceivable that similar evolutionary responses could have occurred in contemporary African hominids, not just in the hominin lineage.
The more anteriorly positioned and horizontally oriented foramen magnum in modern humans compared to extant great apes has been assumed to relate to the more upright posture and bipedal locomotion in hominins16,17,18. However, comparisons with other primates, especially gibbons and short-faced monkeys, suggest that this feature is more broadly associated with differences in head carriage and facial length, rather than uniquely with bipedalism. The distinction between bonobos and chimpanzees in this respect, and the overlap between the morphology of bonobos and that of Sahelanthropus and Ardipithecus further support this contention.
The postcranial evidence for bipedalism in Orrorin and Ardipithecus kadabba mainly involves the dorsal canting of a proximal pedal phalanx (presumed to belong to Ar. kadabba, but from an older geological horizon and with no associated cranio-dental remains)19, and the morphology of the proximal femur in Orrorin20,21. However, these hominin-like postcranial features are also as likely to be functionally and behaviourally associated with arboreal above-branch and terrestrial quadrupedalism as they are with bipedalism22,23. Likewise, the claim that Ardipithecus ramidus was a facultative terrestrial biped is vitiated because it is based on highly speculative inferences about the presence of lumbar lordosis and on relatively few features of the pelvis and foot, many of which also occur in the arboreally adapted Oreopithecus.
Shared morphology need not mean shared history
For much of its history, systematics was predicated on the assumption that there is a direct relationship between morphological similarity and genetic relatedness: that is, the more skeletal morphology two taxa share, the closer their relationship. For extant taxa, this hypothesis can be tested against relationships on the basis of molecular evidence. Such data, either on their own, or in combination with morphological evidence, have been used in efforts to try to resolve relationships among taxa, including those within large clades of medium- to large-sized mammals (for example, refs 24 and 25). But even at this ‘macro’ scale it is apparent that a substantially similar skeletal phenotype does not always mean a shared recent evolutionary history. Long ago, Lankester26 suggested that the term homoplasy be used for morphology that is seen in sister taxa, but not in their MRCA. Such morphology gives the impression the two taxa are more closely related than they really are and because homoplasy can be mistaken for shared derived similarity (or synapomorphy) it complicates attempts to reconstruct relationships.
One could cope with the confounding effects of homoplasy if the ‘noise’ generated by the latter was trivial compared to the strength of the phylogenetic ‘signal.’ But in some attempts to infer relationships among extant higher primates using skeletal data (in the form of either traditional non-metrical characters or characters generated from metrical data) the ratio between ‘noise’ and ‘signal’ was of the order of 1:2. The results of these analyses were not only frustratingly inconclusive, but also when they were compared with the pattern of relationships generated using molecular data, some were misleading27,28. Other researchers suggested that this dismal performance was due to the exclusion of character state data from fossil taxa29, but this argument is moot because soft-tissue characters (for which there are no fossil data) are capable of recovering a pattern of relationships among extant higher primates that is consistent with the molecular evidence30,31. If it is not just the absence of fossils, it must be something about hard-tissue evidence. But thankfully, not all hard-tissue evidence is problematic; it can produce results congruent with the relationships generated from molecular data as long as the anatomical regions targeted have a high enough signal-to-noise ratio32,33. This suggests that the basic problem is with either, or both, the nature of the data or the scale of the enquiry, and not with cladistic methodology. It is not good news for palaeoanthropologists that the type of data the fossil record provides (that is, mostly craniodental hard-tissue morphology) seems to be particularly prone to homoplasy when used at this relatively fine taxonomic level.
There is also comparative evidence for concluding that homoplasy needs to be taken into account when generating hypotheses about the relationships among the taxa in the higher primate part of the tree of life. Although there is overwhelming molecular and morphological evidence for a (((Pan, Homo) Gorilla) Pongo) pattern of relationships among the extant hominids, selected morphological character states can be used to infer a (((Pongo, Homo) Pan) Gorilla) pattern of relationships, but these are almost certainly homoplasies. Similarly, homoplasy complicates attempts to resolve the relationships of fossil apes such as Sivapithecus34, Morotopithecus35,36 and Chororapithecus37. Moreover, studies of other mammalian clades evolving in Africa during the same time period as hominins and in similar palaeoenvironments (such as bovids38, equids39, elephantids40, carnivores41 and Old World monkeys42) point to substantial and recurrent homoplasy. There is no reason to assume that extinct sympatric and synchronic higher primate lineages were immune from the tendency to adapt in similar morphological and phylogenetically confounding ways to similar ecological challenges.
The important point is that shared similarities can only take one so far in determining phylogenetic relationships, because homoplasy, as well as uncertainties in determining the polarity of character transformation, have the potential to generate substantial noise that serves to confound attempts to generate reliable hypotheses about relationships. These considerations have clear implications for generating hypotheses about the phylogenetic position of Ardipithecus, Sahelanthropus and Orrorin. Even if these taxa share some derived features with later Pliocene hominins, it would be rash simply to assume that those features are immune from homoplasy, especially when other aspects of their respective phenotypes are consistent with a more distant relationship with the hominin clade.
Simplicity or complexity in phylogeny
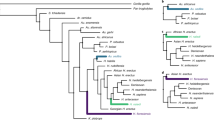
There are two schools of thought about the reconstruction of human evolutionary history. One, which is rooted in the principle of parsimony and takes little or no account of homoplasy around and within the hominin clade, takes the view that the only thing that we ‘know’ is that the evolutionary history of each extant higher primate taxon comprises a series of ancestral–descendant species that can be traced back to the MRCA of all higher primates. While fossil evidence may suggest the presence of extinct clades, such clades are not necessary in the way that direct ancestors of extant taxa are necessary. Thus, the appropriate null hypothesis for such a world-view is that extinct taxa are ancestors of living descendants until proved otherwise. But given that the fossil records of other African mammalian groups document a diversity of extinct lineages with evidence for substantial amounts of homoplasy38,39,40,41,42, an alternative and perhaps more prudent null hypothesis is that a fossil species should only be considered a potential ancestor of a descendant species if its morphology is concordant with the hypothesized ancestral morphotype and lacks any autapomorphies. These different approaches have led to debates in the palaeoanthropological community about whether the shape of human evolutionary history was ladder-like and anagenetic, involving a series of time-successive species43, or more bushy and cladogenetic, with several extinct collateral lineages44 (Fig. 1).
a, According to this hypothesis a 4.4-Myr-old hominid closely related to hominins and panins could only be a hominin ancestor or a panin ancestor. b, According to this hypothesis a 4.4-Myr-old hominid closely related to hominins and panins could be a hominin or a panin ancestor, but is more probably a member of an extinct clade. This hypothesis more closely reflects the comparative evidence.
Comparative evidence points to cladogenesis being the null hypothesis. For example, although a single species of orang-utan, Pongo pygmaeus, occurs today on Borneo and Sumatra (some authorities recognize a second species, Pongo abelii), we know from the fossil record that this is a relict taxon belonging to a diverse radiation of pongins, originating 13 million years ago, that occurred during the Miocene and Plio-Pleistocene45,46. The Eurasian fossil record includes evidence of several extinct subspecies or species of Pongo, as well as a number of Miocene pongine genera (that is, Lufengpithecus, Ankarapithecus, Sivapithecus, Khoratpithecus, and probably Gigantopithecus)45,46,47 (Fig. 2). Climatic and ecological changes during the later Miocene, associated with cooler, more seasonal conditions, coincided with a sharp decline in the diversity of hominids, and by the close of the Miocene pongines had disappeared from much of their former geographical range, surviving only in the subtropical and tropical forests of southeastern Asia46. During the Pleistocene, the range of Pongo extended from southern China through to mainland and island Southeast Asia, but a combination of climatic fluctuations and the arrival of sophisticated human hunters forced a major reduction in the range of orang-utans, eventually leading to their current relictual distribution48.
Grey bars show the time ranges and black lines indicate inferred relationships among the species.
This context is critical to appreciating the apparent diversity of the early purported hominins. Currently, four species are recognized but some researchers have intimated that these taxa might best be subsumed into one or two species of Ardipithecus1,4,43. Obviously, detailed comparisons are needed to resolve these taxonomic issues, but based on published accounts of the morphological differences between the species and what we already know about hominoid diversity during the late Miocene of Eurasia and Africa, it would not be unexpected or surprising to have multiple genera and lineages represented.
Scale in phylogeny reconstruction
Primate systematists generally have no problem using hard-tissue evidence to establish relationships among taxa as disparate as lemurs, Old World monkeys and apes. However, in the case of closely related taxa or stem taxa, the combination of homoplasies, retained primitive features and uncertainties about the polarity of the character sequences used in cladistic analysis generates noise that can overwhelm the modest signal generated by the relatively small number of informative derived characters that separate such taxa from either the MRCA or from stem members of their sister lineage. The problem is one of taxonomic scale; methods that work well for larger taxonomic units may be ineffective or generate misleading results when applied to closely related taxa.
Two examples of uncertainties about the relationships among archaic hominin taxa illustrate the problem of scale. As presently defined, Australopithecus is almost certainly not a monophyletic group, or clade, but a paraphyletic cluster of stem species from which Homo and Paranthropus apparently derive their ancestry. Five species are currently recognized: Au. anamensis, Au. afarensis (including Au. bahrelghazali), Au. africanus, Au. garhi and the recently named Au. sediba49. Of these, Au. anamensis and Au. afarensis are probable time-successive sister taxa50,51, but the relationships of Au. garhi and Au. sediba, as well as the relationship of Australopithecus species to Paranthropus and Homo, are still unresolved. A second example concerns Paranthropus. The vast majority of cladistic analyses of early hominins conclude that the megadont archaic hominins from East and southern Africa (that is, P. boisei and P. robustus) are sister taxa, in which case there are grounds for recognizing that clade as a genus (that is, Paranthropus). However, several independent lines of evidence, both macrostuctural (for example, facial and premolar root morphology)52,53,54 and microstructural (for example, enamel daily secretion rates)55, are not consistent with their being sister taxa. In both of the examples given above the problem arises because the taxa concerned are closely related and adaptively similar. Each species manifests morphology that allows them to be diagnosed as separate taxa, but from a phylogenetic perspective the overlapping combinations of features result in high levels of homoplasy that confound phylogenetic analyses.
So why do researchers persist in trying to solve a phylogenetic problem that may well be at the limits of, or even beyond, the analytical capabilities of the data and the available methods? The reason is that our own ancestry matters to us. Most vertebrate palaeontologists would be content to accept that the ancestry of Homo resides in Australopithecus, without needing or expecting to unravel the topological complexity of the different species within the latter genus. We are not advocating that researchers abandon trying to draw inferences about the phylogenetic relationships of hominins at the finest scale possible. However, we do suggest that those who present and accept these hypotheses need to be aware that such inferences, especially ones about stem taxa, are likely to be inherently prone to refutation and subsequent revision.
Cautionary tales from South Asia and Tuscany
Logic dictates that the earliest hominin taxon should be distinguishable from the reconstructed ancestral morphotype of hominines by at least one derived feature, or synapomorphy, it shares with later hominins. The obvious candidate synapomorphies are those related to facial shortening, encephalization, smaller and more vertically implanted incisors, reduction in the overall size and degree of sexual dimorphism of the canines, modification of the P3 associated with a reduced honing function of the upper canine, postcanine megadontia, as well as specialized features of the vertebral column, pelvis, hindlimb and foot associated with adaptations to upright posture and terrestrial bipedalism. Indeed, the evidence put forward to support the hypothesis that Ar. ramidus belongs to the hominin clade includes its relatively small incisors, small canines exhibiting slight sexual dimorphism, relatively small P3 with a mesiodistally shortened crown, lack of upper canine honing, relatively short face, anteriorly placed foramen magnum and a pelvis with a mediolaterally broad ilium, abbreviated iliac isthmus, inferosuperiorly short pubic symphysis, prominent anterior inferior iliac spine and discrete greater sciatic notch1,56,57. It remains to be seen how many of these alleged hominin synapomorphies withstand close scrutiny, but they are at the crux of the argument in favour of Ardipithecus being a hominin.
Only a quarter of a century ago the palaeoanthropological community learned, or should have learned, a sobering lesson about how easily homoplasy can lead to misinterpretation. The lesson concerns Ramapithecus punjabicus, a late Miocene hominoid from south Asia. Its short face, robust jaws, small canines and thick-enamelled, bunodont molars, were widely interpreted to be evidence that it was an early hominin58. However, the molecular clock implications of the DNA evidence8,9,10, combined with additional fossil material recovered during the 1980s, made it abundantly clear that the fossil evidence for Ramapithecus comprised female specimens of Sivapithecus, a creature that is almost certainly a close relative of the orang-utan45,59.
A further cautionary tale is provided by Oreopithecus bambolii, a late Miocene hominoid from Italy (Fig. 3). Its remains are known from fossil sites in Tuscany and Sardinia that, during the late Miocene (about 7 to 8 million years ago), were located on islands in the northern Mediterranean. Oreopithecus was first described in 1872, but it was not until the discoveries by the Swiss palaeontologist, Johannes Hürzeler, in the late 1950s that a clear appreciation of its anatomy was obtained60,61,62. Although the teeth and skull of Oreopithecus are uniquely specialized, its postcranial skeleton confirms that it is a hominid (that is, a member of the clade that includes the great apes and modern humans)63. Its precise relationships are still debated, but in a number of key features it is more primitive than all extant hominids and is best considered a stem hominid, possibly related to late Miocene hominids from mainland Europe63,64,65.
What is instructive about Oreopithecus with respect to developing hypotheses about the relationships of Ar. ramidus is that it is a species of hominoid that is well-enough known anatomically (that is, almost every bone in the skeleton is represented) to be certain that it is not a member of the hominin clade, yet it shares many anatomical similarities with later hominins, including some that are generally considered to be uniquely associated with bipedal behaviour. The shared similarities include: small and vertically implanted incisors, relatively small canines, a small non-sectorial P3 with a high incidence of a prominent metaconid, absence or small size of a diastema in the upper tooth row, a vertically oriented mandibular symphysis, a mental foramen situated high on the mandibular corpus, a short orthognathic face, an anteriorly placed zygomatic process of the maxilla, anterior projecting nasal apophyses and a deep pit on the palmar aspect of the terminal phalanx of the thumb for attachment of a well developed flexor pollicis longus tendon63,64. Shared similarities associated with bipedal behaviour include an anteriorly situated foramen magnum, short and broad iliac blades, infero-superiorly short pubic symphysis, a well-developed anterior inferior iliac spine, a large ischial spine, medial and lateral condyles of the distal femur similar in size, possibly associated with a bicondylar angle. The impressive suite of shared features with fossil hominins led Hürzeler to deduce (not unreasonably) that Oreopithecus was a fossil hominin62, but these features are most parsimoniously interpreted as either homoplasies or retained primitive hominid features. Oreopithecus is a classic example of how a late Miocene hominid can independently acquire a suite of structural–functional complexes of the dentition, cranium, hand, hip and hindlimb that closely parallel the specialized features uniquely associated with the hominin lineage, and thereby encourage researchers to generate erroneous assumptions about evolutionary relationships. Oreopithecus highlights the dangers inherent in uncritically assuming that shared similarities are a secure indication of relationship or that extant primates are an adequate guide to the potential behavioural diversity of extinct taxa.
The object lesson that Oreopithecus provides is critical to the debate about interpreting the relationships of the earliest purported hominins. It demonstrates how features considered to be hominin specializations can be shown to have been acquired independently in a non-hominin lineage in association with inferred behaviours that are functionally related to, but not necessarily narrowly restricted to, terrestrial bipedalism.
Implications for palaeoanthropology
There are an impressive number of contrasts between the morphology of chimpanzees/bonobos and modern humans, but the differences between the earliest panins and the earliest hominins were fewer and almost certainly more subtle. The working hypothesis has been that whereas the early panins would have had, at some stage in their evolution, projecting faces that accommodated elongated jaws bearing relatively small chewing teeth and large, sexually dimorphic canine teeth, early hominins would have had a masticatory apparatus that combined relatively larger chewing teeth with more modestly sized and less sexually dimorphic canines. As for the locomotor system, it has been hypothesized that early panins were adapted for a combination of arboreal and terrestrial quadrupedalism, vertical climbing and forelimb suspensory behaviours and possibly knuckle-walking, whereas early hominins would show skeletal adaptations for a locomotor strategy that included long bouts of terrestrial bipedalism.
However, this scenario implies that the only option for a 7–4-million-year-old hominid that shares some features with later hominins is that it is a stem hominin. The lessons of Ramapithecus and Oreopithecus suggest that researchers should be skeptical about this assumption. Leaving aside the strong possibility that not all of the purported hominin-like features of Ar. ramidus will withstand critical scrutiny, many of them are also to be found in Oreopithecus and so the possibility that some, or all, of them are primitive hominid features or homoplasies should not be discounted. That Ardipithecus is from Africa (rather than an insular species from the northern Mediterranean), that it has a more conservative craniodental morphology (as opposed to the uniquely specialized skull and teeth of Oreopithecus) and that it chronologically precedes the earliest occurrence of indisputable hominins (Au. anamensis at 4.2 million years ago) does not diminish the likelihood of potential homoplasies. Indeed, one might argue that homoplasies would be more likely given the shared ecogeography and because Ar. ramidus probably has closer phylogenetic ties with early hominins than does Oreopithecus.
This review has dwelt on the probability that homoplasy will confound attempts to identify 7–4-million-year-old hominid taxa as unambiguous early hominins, but the same arguments apply to the evolutionary context of the two associated skeletons of unambiguous hominins recovered from the southern Africa cave site of Malapa and attributed to Au. sediba49. In their case the discussion is complicated by the juvenile status of one of the associated skeletons and an inadequate consideration of intraspecific variation, but the debate is analogous. Instead of ‘hominin versus hominid’ the debate is about whether some features of the cranial (for example, the margins of the orbit) and the postcranial (for example, the ilium) skeleton of the two Malapa skeletons are sufficient evidence to interpret this material as belonging to Homo rather than to Australopithecus. The analogies go deeper, for just as the researchers involved in the analysis of Ar. ramidus make light of the many ways its morphology resembles that of chimpanzees and bonobos (for example, much of the preserved hand and foot morphology of Ar. ramidus is African-ape-like) Berger and colleagues make light of the substantial amount of limb morphology shared between Au. sediba and other Australopithecus taxa.
We emphasize that we are not claiming that the presence of homoplasy in and around the hominin clade, and the other methodological and analytical limitations of phylogenetic analyses noted above, doom all efforts to recover evolutionary relationships to failure. Nor are we claiming that Ar. ramidus, S. tchadensis and O. tugenensis are definitely not hominins. We do, however, advocate that those palaeoanthropologists whose considerable and much valued efforts in the field are rewarded with fossils as significant as those from Aramis, Toros Menalla, Lukeino and Malapa acknowledge the potential shortcomings of their data when it comes to generating hypotheses about relationships. We urge researchers, teachers and students to consider the published phylogenetic interpretations of these taxa as among a number of possible interpretations of the evidence. In the meantime, the rest of us should concentrate on developing methods and approaches that help to discriminate between phylogenetically informative characters and homoplasies. For example, there are new imaging methods providing much better morphological detail66, and some of these methods provide non-destructive access to the microstructure of fossils55,67.
There is no reason why higher primate evolution in Africa in the past ten million years should not mirror the complexity observed in the evolutionary histories of other mammals during the same time period. Nor is there any reason, especially with the lessons from Ramapithecus and Oreopithecus fresh in the minds of researchers, to assume that hominins should not be prone to the same limitations and uncertainties of phylogenetic analysis as other fossil primates. By giving appropriate consideration to parsimony in reconstructing ancestral morphotypes, as well as to the confounding influences of homoplasy and the correct determination of character state polarities, the possibility that one or more of the purported earliest hominins represents a stem hominine or a stem hominid appears to be a plausible, perhaps even preferable, alternative hypothesis. Nevertheless, the fossil evidence from the Aramis, Toros Menalla, Lukeino and Malapa sites, whichever taxonomic and systematic hypotheses survive refutation over the next decade, will continue to provide critical evidence, comparative or otherwise, about the early stages of human evolution.
References
White, T. D. et al. Ardipithecus ramidus and the paleobiology of early hominins. Science 326, 75–86 (2009)
Senut, B. et al. First hominid from the Miocene (Lukeino Formation, Kenya). C. R. Acad. Sci. 332, 137–144 (2001)
Brunet, M. et al. A new hominid from the Upper Miocene of Chad, Central Africa. Nature 418, 145–151 (2002)
Haile-Selassie, Y., Suwa, G. & White, T. D. Late Miocene teeth from Middle Awash, Ethiopia, and early hominid dental evolution. Science 303, 1503–1505 (2004)
Goodman, M. in Classification and Human Evolution (ed. Washburn, S. L.) 204–234 (Aldine, 1963)
Zuckerkandl, E. in Classification and Human Evolution (ed. Washburn, S. L.) 243–272 (Aldine, 1963)
King, M.-C. & Wilson, A. C. Evolution in two levels in humans and chimpanzees. Science 188, 107–116 (1975)
Ruvolo, M. Molecular phylogeny of the hominoids: inferences from multiple independent DNA sequence data sets. Mol. Biol. Evol. 14, 248–265 (1997)
Bradley, B. Reconstructing phylogenies and phenotypes: a molecular view of human evolution. J. Anat. 212, 337–353 (2008)
Patterson, N. et al. Genetic evidence for complex speciation of humans and chimpanzees. Nature 441, 1103–1108 (2006)
Fabre, P.-H., Rodrigues, A. & Douzery, E. J. P. Patterns of macroevolution among primates inferred from a supermatrix of mitochondrial and nuclear DNA. Mol. Phyl. Evol. 53, 808–825 (2009)
Werdelin, L., Sanders, W. J., eds. Cenozoic Mammals of Africa (University of California Press, 2010)
Pilbeam, D. R. in The Primate Fossil Record (ed. Hartwig, W. C.) 303–310 (Cambridge University Press, 2002)
Andrews, P. & Harrison, T. in Interpreting the Past: Essays on Human, Primate, and Mammal Evolution in Honor of David Pilbeam (eds Lieberman, D. E., Smith, R. J. & Kelley, J.) 103–121 (Brill, 2005)
Shreeve, J. 4.4 Million years ago: the birth of bipedalism. Nat. Geogr. Mag. 218, 63–66 (2010)
White, T. D., Suwa, G. & Asfaw, B. Australopithecus ramidus, a new species of hominid from Aramis, Ethiopia. Nature 371, 306–312 (1994)
Suwa, G. et al. The Ardipithecus ramidus skull and its implications for hominid origins. Science 326, 68e1–68e7 (2009)
Zollikofer, C. P. E. et al. Virtual cranial reconstruction of Sahelanthropus tchadensis . Nature 434, 755–759 (2005)
Haile-Selassie, Y. Late Miocene hominids from the Middle Awash, Ethiopia. Nature 412, 178–181 (2001)
Galik, K. et al. External and internal morphology of the BAR 1002’00 Orrorin tugenensis femur. Science 305, 1450–1453 (2004)
Richmond, B. G. & Jungers, W. L. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science 319, 1662–1665 (2008)
Rein, T. R. & Harrison, T. Quantifying the angle of orientation of the metatarsophalangeal joint surface of proximal phalanges in extant primates. Am. J. Phys. Anthropol. 132 (S44). 197 (2007)
Rafferty, K. L. Structural design of the femoral neck in primates. J. Hum. Evol. 34, 361–383 (1998)
Fernández, M. H. & Vrba, E. S. A complete estimate of the phylogenetic relationships in Ruminantia: a dated species-level supertree of extant ruminants. Biol. Rev. Camb. Philos. Soc. 80, 269–302 (2005)
Flynn, J. J. et al. Molecular phylogeny of the Carnivora (Mammalia): assessing the impact of increased sampling on resolving enigmatic relationships. Syst. Biol. 54, 317–337 (2005)
Lankester, E. R. On the use of the term homology. Ann. Mag. Nat. Hist. Zool. Botany Geol. 6, 34–43 (1870)
Collard, M. & Wood, B. Hominin homoiology: an assessment of the impact of phenotypic plasticity on phylogenetic analyses of humans and their fossil relatives. J. Hum. Evol. 52, 573–584 (2007)
Collard, M. & Wood, B. How reliable are human phylogenetic hypotheses? Proc. Natl Acad. Sci. USA 97, 5003–5006 (2000)This paper showed that when conventional metrical and non-metrical hard-tissue characters are used to generate hypotheses about the relationships among the great apes and the baboon/mangabey group the resulting cladograms are not consistent with the pattern of relationships supported by molecular evidence.
Strait, D. S. & Grine, F. E. Inferring hominoid and early hominid phylogeny using craniodental characters: the role of fossil taxa. J. Hum. Evol. 47, 399–452 (2004)
Gibbs, S., Collard, M. & Wood, B. Soft-tissue characters in higher primate phylogenetics. Proc. Natl Acad. Sci. USA 97, 11130–11132 (2000)This paper showed that in contrast to the poor performance of hard-tissue characters when soft-tissue characters are used to generate hypotheses about the relationships among the great apes the resulting cladograms are consistent with the pattern of relationships supported by molecular evidence.
Gibbs, S., Collard, M. & Wood, B. Soft-tissue anatomy of the extant hominoids: a review and phylogenetic analysis. J. Anat. 200, 3–49 (2002)
Lockwood, C. A., Kimbel, W. H. & Lynch, J. M. Morphometrics and hominoid phylogeny: support for a chimpanzee-human clade and differentiation among great ape subspecies. Proc. Natl Acad. Sci. USA 101, 4356–4360 (2004)
Harvati, K. & Weaver, T. D. in Neanderthals Revisited: New Approaches and Perspectives (eds Harvati, K. & Harrison, T.) 239–254 (Springer, 2006)
Young, N. M. A reassessment of living hominoid postcranial variability: implications for ape evolution. J. Hum. Evol. 45, 441–464 (2003)
Harrison, T. in Cenozoic Mammals of Africa (eds Werdelin, L. & Sanders, W. J.) 429–469 (University of California Press, 2010)
Nakatsukasa, M. Comparative study of Moroto vertebral specimens. J. Hum. Evol. 55, 581–588 (2008)
Suwa, G. et al. A new species of great ape from the late Miocene epoch in Ethiopia. Nature 448, 921–924 (2007)
Gatesy, J. et al. A cladistic analysis of mitochondrial ribosomal DNA from the Bovidae. Mol. Phyl. Evol. 7, 303–319 (1997)
Bernor, R. L. et al. in Cenozoic Mammals of Africa (eds Werdelin, L. & Sanders, W. J.) 685–721 (University of California Press, 2010)
Todd, N. E. New phylogenetic analysis of the family Elephantidae based on cranio-dental morphology. Anat. Rec. 293, 74–90 (2010)
Van Valkenburgh, B. Déjà vu: the evolution of feeding morphologies in the Carnivora. Integr. Comp. Biol. 47, 147–163 (2007)
Jablonski N. G., Leakey M. G., eds. Koobi Fora Research Project Vol. 6 The Fossil Monkeys (California Academy of Science, 2008)
White, T. D. in The Paleobiological Revolution: Essays on the Growth of Modern Paleontology (eds Sepkoski, D. & Ruse, M.) 122–148 (University of Chicago Press, 2009)
Wood, B. A. Reconstructing human evolution: achievements, challenges and opportunities. Proc. Natl Acad. Sci. USA 107 (Suppl. 2). 8902–8909 (2010)
Kelley, J. in The Primate Fossil Record (ed. Hartwig, W. C.) 369–384 (Cambridge Univesrity Press, 2002)
Harrison, T. Apes among the tangled branches of human origins. Science 327, 532–534 (2010)This paper shows that there is a remarkable diversity of fossil apes from the Miocene that represents precursors of the hominins and highlights the uncertainties in interpreting the phylogenetic placement of the earliest purported hominins.
Harrison, T., Ji, X. & Su, D. On the systematic status of the late Miocene and Pliocene hominoids from Yunnan Province, China. J. Hum. Evol. 43, 207–227 (2002)
Harrison, T., Krigbaum, J. S. & Manser, J. in Primate Biogeography (eds Fleagle, J. G. & Lehman, S. M.) 323–364 (Springer, 2006)
Berger, L. R. et al. Australopithecus sediba: a new species of Homo-like australopith from South Africa. Science 328, 195–204 (2010)
Lockwood, C. A., Kimbel, W. H. & Johanson, D. C. Temporal trends and metric variation in the mandibles and dentition of Australopithecus afarensis . J. Hum. Evol. 39, 23–55 (2000)
Kimbel, W. H. & Delezene, L. K. “Lucy” redux: a review of research on Australopithecus afarensis . Ybk Phys. Anthropol. 140 (49). 2–48 (2009)
Rak, Y. The Australopithecine Face (Academic Press, 1983)
Wood, B. A. in Evolutionary History of the “Robust” Australopithecines (ed. Grine, F. E.) 269–284 (Aldine de Gruyter, 1988)
McCollum, M. The robust australopithecine face: a morphometric perspective. Science 284, 301–305 (1999)
Lacruz, R. S., Dean, M. C., Ramirez-Rossi, F. & Bromage, T. G. Megadontia, striae periodicity and patterns of enamel secretion in Plio-Pleistocene fossil hominins. J. Anat. 213, 148–158 (2008)
Lovejoy, C. O. et al. The pelvis and femur of Ardipithecus ramidus: the emergence of upright walking. Science 326, 71e1–71e6 (2009)
Suwa, G. et al. Paleobiological implications of the Ardipithecus ramidus dentition. Science 326, 94–99 (2009)
Simons, E. L. The phyletic position of Ramapithecus . Postilla 54, 1–20 (1961)
Pilbeam, D. R. New hominoid skull material from the Miocene of Pakistan. Nature 295, 232–234 (1982)
Hürzeler, J. Zur systematischen Stellung von Oreopithecus . Verh. Naturf. Ges. Basel 65, 88–95 (1954)
Straus, W. L. in Classification and Human Evolution (ed. Washburn, S. L.) 146–177 (Aldine, 1963)
Hürzeler, J. Oreopithecus bambolii Gervais: a preliminary report. Verh. Naturf. Ges. Basel 69, 1–48 (1958)
Harrison, T. & Rook, L. in Function, Phylogeny and Fossils: Miocene Hominoid Evolution and Adaptation (eds Begun, D. R., Ward, C. V. & Rose, M. D.) 327–362 (Plenum, 1997)This is a detailed study of the anatomy and phylogenetic relationships of Oreopithecus bambolii , demonstrating that it is a stem hominid with many postcranial features that parallel the specialized anatomy of modern humans.
Moyà Solà, S. & Köhler, M. The phylogenetic relationships of Oreopithecus bambolii Gervais, 1872. C. R. Acad. Sci. Paris 324 (sér. IIa). 141–148 (1997)
Sarmiento, E. E. The phylogenetic position of Oreopithecus and its significance in the origin of the Hominoidea. Am. Mus. Novit. 2881, 1–44 (1987)
Skinner, M., Wood, B. A. & Hublin, J.-J. Enamel-dentine junction (EDJ) morphology distinguishes the lower molars of Australopithecus africanus and Paranthropus robustus . J. Hum. Evol. 55, 979–988 (2008)
Smith, T. & Tafforeau, P. New visions of dental tissue research: tooth development, chemistry, and structure. Evol. Anthropol. 17, 213–226 (2008)
Acknowledgements
Support was provided by the GW Vice-President for Academic Affairs and to the GW Selective Excellence Program (to Provost and B.W.) and the NSF (BCS-0309513) (to T.H.). We thank R. Bernstein, J. DeSilva, T. Kivell, D. Pilbeam and B. Richmond for their critical comments and suggestions.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to the research and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wood, B., Harrison, T. The evolutionary context of the first hominins. Nature 470, 347–352 (2011). https://doi.org/10.1038/nature09709
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09709
This article is cited by
-
Palaeobotanical evidence reveals the living conditions of Miocene Lufengpithecus in East Asia
BMC Plant Biology (2023)
-
Reappraising the palaeobiology of Australopithecus
Nature (2023)
-
Postcranial evidence of late Miocene hominin bipedalism in Chad
Nature (2022)
-
Standing up for the earliest bipedal hominins
Nature (2022)
-
Growth and development of the third permanent molar in Paranthropus robustus from Swartkrans, South Africa
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.