Abstract
Breast cancer is one of the most common cancers in humans and will on average affect up to one in eight women in their lifetime in the United States and Europe1. The Women’s Health Initiative and the Million Women Study have shown that hormone replacement therapy is associated with an increased risk of incident and fatal breast cancer2,3. In particular, synthetic progesterone derivatives (progestins) such as medroxyprogesterone acetate (MPA), used in millions of women for hormone replacement therapy and contraceptives, markedly increase the risk of developing breast cancer. Here we show that the in vivo administration of MPA triggers massive induction of the key osteoclast differentiation factor RANKL (receptor activator of NF-κB ligand) in mammary-gland epithelial cells. Genetic inactivation of the RANKL receptor RANK in mammary-gland epithelial cells prevents MPA-induced epithelial proliferation, impairs expansion of the CD49fhi stem-cell-enriched population, and sensitizes these cells to DNA-damage-induced cell death. Deletion of RANK from the mammary epithelium results in a markedly decreased incidence and delayed onset of MPA-driven mammary cancer. These data show that the RANKL/RANK system controls the incidence and onset of progestin-driven breast cancer.
Similar content being viewed by others
Main
RANKL (also known as ODF, TRANCE, OPGL and TNFSF11) and its receptor RANK (also known as TRANCE-R and TNFRSF11A) are essential for the development and activation of osteoclasts4,5. The RANKL/RANK system also controls lymph-node organogenesis, the development of thymic medullary epithelial cells4,5,6, central thermoregulation7 and the formation of a lactating mammary gland during pregnancy8. The expression of both RANKL and RANK has been observed in primary breast cancers in humans and breast cancer cell lines9, and we and others have proposed that the RANKL/RANK system can regulate bone metastases of epithelial tumours9,10. On the basis of the clinical importance of RANKL inhibition being approved for potentially millions of patients to prevent bone loss11, the genetically defined role of RANKL/RANK in mammary epithelial proliferation in pregnancy8 and the regulation of the RANKL/RANK system by sex hormones8,12, we speculated that RANKL/RANK might affect the development of primary, hormone-driven mammary cancer.
Progesterone and its synthetic derivatives (progestins) are used in combined hormone replacement therapy in postmenopausal women13. MPA is the most frequently and longest used progestin in hormonal contraceptives14 and is commonly used for hormone replacement therapy15. Because we8 and others12 have previously shown that progesterone can induce RANKL in vivo, we tested whether MPA can also induce RANKL expression. Treatment with MPA resulted in a more than 2,000-fold induction of messenger RNA encoding RANKL in isolated mammary epithelial cells (Fig. 1a). Expression of mRNA encoding RANK, osteoprogesterin16, PTHrP (parathyroid-hormone-related protein) and TRAIL (TNF-related apoptosis-inducing ligand)16 were not changed. Immunohistochemistry confirmed the induction of RANKL protein in the mammary gland (Fig. 1b). Western blotting showed induction of the 19-kDa soluble form of RANKL (Supplementary Fig. 1a), most probably resulting from alternative splicing (Supplementary Fig. 1b–d) as well as induction of matrix metalloproteinase 14 and ADAM17 (a disintegrin and metalloproteinase 17, also called TACE (tumour-necrosis-factor-α-converting enzyme) (Supplementary Fig. 1e), two proteases known to shed RANKL16. In addition, MPA might induce mammary-gland prolactin receptor, which then triggers RANKL by means of Jak-2/STAT5 signalling17. Whereas MPA triggers the substantial production of RANKL protein in female control mice, we failed to detect RANKL induction in prolactin receptor mutants (Supplementary Fig. 1f). Thus, MPA, through progesterone receptors, triggers massive RANKL expression in the mammary gland.
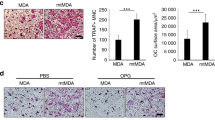
a, b, Induction of RANKL expression by the progesterone derivative MPA. Nulliparous wild-type females were implanted subcutaneously with slow-release MPA pellets or treated with sham surgery. a, mRNA encoding RANKL was determined in purified mammary epithelial cells by quantitative RT–PCR three days after implantation. β-Actin mRNA was used for normalization. Data are shown as fold change compared with sham treatment (means ± s.e.m., n = 3). b, In situ immunostaining of progesterone receptor (PR, red) and RANKL (green) in mammary epithelial cells after treatment with MPA for 3 days. c, Epithelial proliferation in mammary glands of control littermates and RANKΔmam females 3 days after sham treatment and MPA implantation. Proliferation was determined by in situ Ki67 immunostaining. d, Marked increase in the stem-cell-enriched CD24+CD49fhi population (MaSC) in MPA-treated mammary glands in control but not in RANKΔmam mammary glands. Representative FACS profiles showing CD24 and CD49f expression of lineage-negative (CD31− (endothelial cells) CD45− (haematopoietic cells) TER199− (erythroid cells)) mammary MaSCs from MPA-treated or sham-treated eight-week-old virgin females. Asterisk, P < 0.05; three asterisks, P < 0.001 (Student’s t-test).
To examine the potential role of RANKL/RANK in MPA-mediated tumorigenesis we generated K5-Cre rankflox/Δ mice and murine-mammary-tumour virus (MMTV)-Cre rankflox/Δ mice to delete RANK from mammary epithelial cells7. Both mouse lines seemed healthy. As expected from our previous studies on whole-body RANKL and RANK mutants4,8, K5-Cre rankflox/Δ mice showed normal development of mammary glands in puberty; however, these mice did not develop milk-secreting structures during pregnancy (Supplementary Fig. 2a–c). In MMTV-Cre rankflox/Δ mice, the formation of milk-secreting lobuloalveolar structures in pregnancy seemed normal (Supplementary Fig. 3a–c). To exclude any issue of development effects in K5-Cre rankflox/Δ mice we therefore used MMTV-Cre rankflox/Δ mice. These MMTV-Cre rankflox/Δ mutant mice are hereafter termed RANKΔmam.
Treatment with MPA triggers a massive proliferation of mammary epithelial cells18. MPA-induced proliferation of mammary epithelial cells was significantly decreased in RANKΔmam females (Fig. 1c and Supplementary Fig. 4a–c). Accordingly, intraperitoneal RANKL injections into nulliparous females triggered the proliferation of mammary-gland epithelial cells through RANK (Supplementary Fig. 4d, e). Endogenous progesterone can affect the numbers of Lin-CD24+CD49fhi stem cells during pregnancy19 and the oestrous cycle20. In these studies the RANKL/RANK system was implicated; however, it is not known whether this is a direct effect. Treatment with MPA resulted in a twofold expansion of Lin-CD24+CD49fhi cells. This expansion did not occur in MPA-treated RANKΔmam females (Fig. 1d and Supplementary Fig. 4f). These data provide genetic proof that the RANKL/RANK system controls the expansion of Lin-CD24+CD49fhi cells.
In the Women’s Health Initiative and the Million Women Study, the use of progestins has been linked epidemiologically to the onset and incidence of breast cancer2,3. Because RANKL is induced by MPA in vivo, we speculated that RANKL/RANK might affect sex-hormone-driven mammary cancer. Progestin-driven mammary cancer can be modelled in female mice18, in which the implantation of slow-release MPA pellets in the presence of the DNA-damaging agent 7,12-dimethylbenz[a]anthracene (DMBA) triggers mammary cancer (Fig. 2a and Supplementary Fig. 5a, b). In control females, treatment with MPA and DMBA induced a rapid onset of palpable mammary tumours. In RANKΔmam females we observed a marked delay in the onset of mammary cancer induced by MPA and DMBA (Fig. 2b and Supplementary Fig. 5c, d) and markedly enhanced survival (Supplementary Fig. 5e). Southern blotting of the tumours that developed in RANKΔmam females confirmed the efficient deletion of RANK (Supplementary Information and Supplementary Fig. 5c). All tumours that developed in control and RANKΔmam females showed a histopathology typical of E-cadherin+ ductal adenocarcinomas of the cytokeratin5+ (CK5+) and CK14+ basal cell subtype (Fig. 2c and Supplementary Fig. 6a, b). Ductal adenocarcinomas arising in RANKΔmam females frequently developed extensive areas of squamous metaplasia (Fig. 2c and Supplementary Fig. 6a, b). In line with these histopathological changes, gene-expression profiling of mammary carcinomas from control and RANKΔmam females showed distinctive differences in their molecular signatures as assessed by gene clustering (Supplementary Information and Supplementary Fig. 7a–c).
a, Carcinogenesis scheme involving MPA and DMBA. Nulliparous six-week-old female mice were implanted subcutaneously with MPA pellets and treated orally with DMBA as indicated for eight weeks. b, Onset of palpable mammary tumours in MMTV-Cre rankfloxed/Δ females (RANKΔmam) (n = 14) and age-matched littermate control females (n = 19) treated with MPA pellets and DMBA as indicated in a. Data are shown as the percentage of tumour-free mice after the last DMBA challenge. Median tumour onset for controls was 11 days after the last DMBA treatment; for RANKΔmam females it was 30 days. c, Representative histological sections of mammary tumours isolated from control littermate and RANKΔmam females 22 days after the last DMBA treatment. Cytokeratin 5 staining is shown. Original magnifications ×20. d, e, Numbers of carcinomas in situ and invasive mammary cancers in control and RANKΔmam females on day 7 (d) and day 22 (e) after the final DMBA treatment. Data are shown as means ± s.e.m. per mouse (n = 3 mice per genotype). All ten mammary glands were analysed for each mouse. Asterisk, P < 0.05 (Student’s t-test). Bottom panels show representative histological sections with typical invasive adenocarcinomas in the control females. For RANKΔmam females, normal acinar morphology (day 7) and a carcinoma in situ (day 22) are shown. Haematoxylin/eosin (H&E)-stained sections and immunostaining for the proliferation marker Ki67 are shown. Original magnifications ×20.
One week after the last DMBA treatment, all RANK-expressing control females already showed multiple in situ carcinomas and even invasive mammary tumours. By contrast, at this time point we observed very few carcinomas in situ and never any invasive mammary carcinomas in RANKΔmam animals (Fig. 2d). Three weeks after the last DMBA challenge, the incidence of carcinomas in situ was similar in control and RANKΔmam females, but invasive carcinomas were still infrequent in the RANKΔmam mice (Fig. 2e). Proliferation was typically decreased in tumours from RANKΔmam females (Fig. 2d, e). Deletion of RANK in multiple other tissues including the liver, heart, muscle and the haematopoietic compartment, but not in mammary epithelial cells, using Mx-Cre rankflox/flox mice did not delay the onset of mammary cancer induced by MPA and DMBA (Supplementary Fig. 8a–c), suggesting that the effects of RANK deletion are restricted to mammary epithelial cells. In MMTV-NeuT transgenic mice, mammary-gland-specific deletion of RANK did not alter the incidence of mammary cancer (Supplementary Fig. 8d, e) or the histopathology of adenocarcinomas (Supplementary Fig. 9a–c). In NeuT tumours we observed very low levels of RANKL (Supplementary Fig. 9d, e). However, we cannot exclude effects of the RANKL/RANK system on some aspects of NeuT-driven tumorigenesis, for example metastatic behaviour. Thus, genetic inactivation of RANK in mammary epithelial cells results in a markedly delayed onset and decreased incidence of progestin-driven mammary cancer.
Previously, we8 and others21 have shown that the RANKL/RANK system signals through IκB kinase-α (IKK-α)–NF-κB–cyclin D1 in mammary epithelial cells (Supplementary Fig. 10a). Stimulation with RANKL did indeed result in p65 NF-κB and IκB-α phosphorylation in mouse primary mammary epithelial cells (MECs) (Fig. 3a). In addition, stimulation of MECs with RANKL triggered phosphorylation of p38 mitogen-activated protein kinase (MAPKs) and extracellular signal-regulated kinase (ERK) (Supplementary Fig. 10b). A three-day in vivo treatment with MPA resulted in the accumulation of phosphorylated IkB-α in the nucleus, indicative of an active NF-κB pathway22, and the induction of cyclin D1 protein expression in mammary epithelial cells, both of which were severely decreased in RANKΔmam females (Supplementary Fig. 11a, b). Moreover, in MPA/DMBA-induced mammary adenocarcinomas from control and RANKΔmam females we found impaired activation of the NF-κB pathway (Fig. 3b) and downregulated mRNA expression of cyclin D1 (Fig. 3c). In these primary tumours we also observed upregulation of p21 mRNA (Fig. 3c), a gene that is transcriptionally suppressed by the Id2 pathway23. The Id2 pathway is a second genetically confirmed downstream pathway for RANKL/RANK in mammary epithelial cells23. To extend these findings to humans we assayed SKBR3 breast tumour cells. Stimulation with RANKL induced NF-κB, p38 MAPKs and ERK activation and proliferation in SKBR3 cells (Supplementary Fig. 12a, b). We next assessed the ability of these cells to grow in an anchorage-independent manner, which correlates well with tumorigenicity in vivo24. In a similar manner to stimulation with epidermal growth factor receptor, we observed that RANKL induced the growth of SKBR3 cells in soft agar (Fig. 3d); that is, RANK signalling not only triggers proliferation but also acts as a transforming agent to induce anchorage-independent growth.
a, Western blotting for phosphorylated (P) IKK-α, total IKK-α, phosphorylated p65 NF-κB, total p65 NF-κB, phosphorylated IκB-α, and total IκB-α in isolated MECs in response to stimulation with RANKL (1 μg ml−1). β-Actin is shown as loading control. b, Western blotting for IKK-α, IKK-β, IKK-γ, phosphorylated p65 NF-κB, total p65 NF-κB, phosphorylated IκB-α and total IκB-α in pooled late-stage mammary adenocarcinomas (n = 4 for each lane) that developed in control, RANKΔmam and IKK-αΔmam females. β-Actin is shown as loading control. c, Expression of mRNA encoding RANK, cyclin D1 and p21 in late-stage mammary adenocarcinomas that developed in control, RANKΔmam and IKK-αΔmam females. Expression was determined by quantitative RT–PCR. β-Actin mRNA was used for normalization. Data are means ± s.e.m. (n = 4 per group). d, Soft-agar colony formation assay. Growth of human SKBR3 breast cancer cells in soft agar in response to stimulation with RANKL (1 μg ml−1) or epidermal growth factor (100 ng ml−1). Anchorage-independent macroscopic colonies formed after 18 days in culture with RANKL, which was prevented by the decoy receptor osteoprogesterin (OPG) (1 μg ml−1). Controls were unstimulated SKBR3 cells. e, Onset of palpable mammary tumours in IKK-αΔmam (n = 10) and age-matched littermate control (n = 11) females treated with MPA pellets and DMBA. Data are shown as percentages of tumour-free mice after the last DMBA challenge. Median tumour onset for controls was 10 days after the last DMBA treatment, and for IKK-αΔmam females it was 24 days.
In osteoclasts, besides the NF-κB pathway, the calcineurin-NFATc1 signalling pathway is essential for RANKL/RANK-mediated osteoclastogenesis16. NFATc1 can also be regulated by the Id2 pathway during RANKL-mediated osteoclastogenesis25. We therefore generated MMTV-Cre nfatc1flox/Δ (NFATc1Δmam) and MMTV-Cre Ikkαflox/flox (IKK-αΔmam) mice to delete NFATc1 and IKK-α in mammary epithelial cells. Both mutant mouse strains appear healthy. When challenged with MPA and DMBA, IKK-αΔmam mice showed a delayed onset of mammary cancer (Fig. 3e). In tumours from IKK-αΔmam mice we found normal expression of IKK-β and IKK-γ but decreased NF-κB activation as determined by increased IκB protein levels and decreased p65 NF-κB phosphorylation (Fig. 3b) and downregulated mRNA expression of the NF-κB target gene encoding cyclin D1 (Fig. 3c). As expected, the Id2 pathway gene p21 was not affected in tumours from IKK-αΔmam mice (Fig. 3c). We did not observe any significant differences in tumour onset between control and NFATc1Δmam mice (Supplementary Fig. 12c, d), suggesting that downstream signalling requirements are different in osteoclast progenitors and mammary-gland epithelial cells. Thus, deletion of IKK-α, but not NFATc1, in mammary-gland epithelial cells affects the onset of progestin-driven mammary cancer.
Although treatment with MPA induces very rapid and massive proliferation of mammary epithelial cells, MPA alone is not sufficient to trigger mammary cancer, which requires a carcinogen to induce DNA mutations18. To analyse the role of RANKL in the cellular response to DNA damage, we treated MECs and the RANKL-responsive human breast cancer cell line SKBR3 with the DNA-damaging agents doxorubicin or γ-irradiation. Treatment with RANKL did not alter the induction of γ-H2AX and p53 or the activation of Chk1, prototypic markers of a functional DNA-damage response (Supplementary Fig. 13a). Moreover, RANKL did not alter the early cell-cycle arrest after DNA damage with γ-irradiation (Supplementary Fig. 13b, c) or doxorubicin (Supplementary Fig. 14a, b). However, treatment with RANKL resulted in marked protection from cell death in response to γ-irradiation (Supplementary Fig. 13b, c) and doxorubicin (Supplementary Fig. 14a, b). Mechanistically, γ-irradiation-induced upregulation of the pro-apoptotic molecules Bim, Puma and Noxa did not occur in the presence of RANKL (Supplementary Fig. 14c). We next assessed the effects of the MPA–RANKL/RANK axis on γ-irradiation-induced apoptosis of mammary epithelial cells26. Treatment with MPA did indeed give protection from γ-irradiation-induced apoptosis of mammary epithelial cells in vivo. Loss of RANK expression on mammary epithelial cells abrogated the protective effects of MPA on γ-irradiation-induced cell death (Fig. 4a, b). Moreover, the IKK-α pathway is involved in MPA-induced protection of the mammary epithelium after γ-irradiation (Supplementary Fig. 15a, b). Thus, in addition to promoting cell-cycle progression, MPA can protect from cell death after DNA damage, by means of RANKL/RANK and IKK-α signalling.
a, γ-Irradiation (5 Gy) induced mammary epithelial cell apoptosis in control and RANKΔmam female littermates either sham operated or implanted with a pellet of MPA. Apoptosis was detected by immunostaining for active caspase-3. Apoptotic nuclei of epithelial cells (arrows) are shown for representative mammary-gland sections. Original magnifications ×40. b, Quantification of mammary epithelial apoptosis. A minimum of 5,000 nuclei were counted for each mouse. Results shown are means ± s.e.m. (n = 3 mice per group). Two asterisks, P < 0.02 (Student’s t-test). c, Self-renewal of mammary cancer stem cells (TICs) requires RANK expression. Mammary tumour cells from RANKΔmam and control littermate females treated with MPA and DMBA were cultured for 7 days; a small percentage of primary cells formed mammospheres (first passage). Primary mammospheres were then digested into single cells and assayed for their ability to form secondary mammospheres (second passage). Tumour cells from control littermate but not from RANKΔmam females could form secondary mammospheres determined 7 days after replating. d, Quantification of primary and secondary passage (F1 and F2) mammospheres from RANKΔmam and control littermate females treated with MPA and DMBA. Results shown are means ± s.e.m. (n = 3 mice per group). Asterisk, P < 0.05 (Student’s t-test).
Recently it has been shown in humans and mice that mammary tumours might arise from stem-cell populations27. We therefore tested whether a loss of RANK affects mammary cancer stem cells; that is, tumour-initiating cells (TICs). TICs can be functionally assayed by their ability to form non-adherent mammospheres27,28. Freshly isolated cancer cells from control and RANKΔmam females were able to form primary mammospheres; however, after dispersion into single cells the ability to form secondary mammospheres was significantly impaired when TICs from RANKΔmam mice were used (Fig. 4c, d). These data indicate that a loss of RANK expression markedly impairs the self-renewal capacity of putative cancer stem cells.
On the basis of our results we propose the following molecular model to explain how progestins such as MPA drive mammary cancer: MPA triggers an enormous induction of RANKL in the mammary gland. RANKL, through RANK on mammary epithelial cells, drives these cells into the cell cycle and protects mouse and human mammary-gland epithelial cells from apoptosis in response to DNA damage. Moreover, the RANKL/RANK system controls self-renewal of TICs (defined by mammosphere assays) and anchorage-independent growth in vitro. Thus, the progestin-induced RANKL/RANK system provides a growth and survival advantage to damaged mammary epithelium, a prerequisite to the initiation of mammary cancer29. These effects seem to be mediated, at least in part, by IKK-α–NF-κB signalling.
These data have some intriguing implications. For instance, progestins have been associated with an increased risk of having an abnormal mammogram30. Because mammograms detect microcalcifications and glandular density30, and RANKL/RANK are crucial in bone metabolism4,5, one could speculate that the RANKL/RANK system contributes to the calcification of such lesions and/or glandular densities. Millions of women take progesterone derivatives in contraceptives and for hormone replacement therapy. Because our results show that the RANKL/RANK system is an important molecular link between progestins and epithelial carcinogenesis, RANKL inhibition should be considered as a novel approach to the prevention and/or treatment of breast cancer.
Methods Summary
Mice
Mice carrying the rankflox allele, and MMTV-Cre, MMTV-NeuT, Mx-Cre, K5-Cre, IKKαflox and NFATc1flox mice have been described previously (see Methods). In all experiments, only littermate mice from the same breedings were used. Mice were bred and maintained in accordance with institutional guidelines.
MPA and DMBA-induced mammary carcinogenesis
Treatment with MPA and DMBA was performed as described18,28. Six-week-old female mice were anaesthetized with ketamine–xylazine and subcutaneously implanted with slow-release MPA pellets (50 mg, 90-day release). DMBA (200 μl; 5 mg ml−1) was administered by oral gavage six times throughout the following eight weeks as outlined in Fig. 2a. Onset of mammary tumours was determined by palpation. We did not observe differences in tumour onset in Cre-negative control females and littermates expressing the MMTV-Cre transgene, indicating that Cre expression does not itself alter tumour incidence in the MPA and DMBA mammary tumour model.
Fluorescence-activated cell sorting (FACS) analysis of primary mammary epithelial cells
Purified mammary epithelial cells were stained with the following antibodies: biotin-conjugated anti-CD31 (catalogue no. 553371; BD), which labels endothelial cells, and biotinylated CD45+ and Ter119+ (StemSep murine chimaera cocktail, catalogue no. 13058C; Stem Cell Technologies) both of which label haematopoietic cells. Haematopoietic and endothelial cells were excluded by FACS with streptavidin-conjugated allophycocyanin (catalogue no. 554067; BD). Staining with anti-CD49f (catalogue no. 551129; BD) and CD24 (catalogue no. 553261; BD) was used to identify the mammary-stem-cell-enriched population37,38.
Mammosphere assays
Similar-sized tumours (1 cm3 volume) were digested in complete EpiCult medium with 2.5 × collagenase/hyaluronidase. Cells (2 × 105) were then cultured in serum-free EpiCult medium supplemented with B27 (Invitrogen), 20 ng ml−1 epidermal growth factor (Protech) and 20 ng ml−1 basic fibroblast growth factor (Sigma), using ultra-low-attachment plates. Primary mammospheres were collected on day 7, digested into single-cell suspensions with trypsin, and assayed for their ability to form secondary mammospheres28,39.
Online Methods
Mice
rankflox mice have recently been generated in our laboratory7. MMTV-Cre31, MMTV-NeuT32, Mx-Cre33, K5-Cre34, IKKαflox (ref. 35) and NFATc1flox (ref. 36) mice have been described previously In brief, to generate mice carrying a null allele of rank (rankΔ allele), rankflox mice were crossed to β-actin-Cre ubiquitous deleter mice. Mice carrying the rankflox or rankΔ alleles and also the MMTV-Cre mice were backcrossed seven times onto a BALBc background before generating the MMTV-Cre rankΔ/flox mice. MMTV-NeuT mice were provided by G. Forni. MMTV-Cre (stock no. 003553) and Mx-Cre mice (stock no. 003556) were obtained from the Jackson Laboratory. Mouse genotypes were determined by PCR and Southern blot analysis. In all experiments described in this paper, only littermate mice from the same breedings were used. All mice were bred and maintained in accordance with institutional guidelines.
Mammary carcinogenesis induced with MPA and DMBA
Treatment with MPA and DMBA was performed as described18,28. In brief, six-week-old female mice were anaesthetized with ketamine–xylazine and surgically implanted with slow-release MPA pellets (50 mg, 90-day release; Innovative Research of America) subcutaneously on the right flank. DMBA (200 μl; 5 mg ml−1 diluted in cottonseed oil) was administered by oral gavage six times throughout the following eight weeks as outlined in Fig. 2a. Onset of mammary tumours was determined by palpation. We did not observe differences in tumour onset in Cre-negative control females and littermates expressing the MMTV-Cre transgene, indicating that Cre expression does not itself alter tumour incidence in the MPA and DMBA mammary tumour model.
Mammary tissue transplants
For transplantation studies, mammary epithelial tissue was isolated from nulliparous three-week-old donors and implanted into cleared mammary fat pads (devoid of endogenous epithelium) of three-week-old host nu/nu mice as described40. Cleared fat-pad transplantation is a standard technique in mammary-gland biology to assess the cell-autonomous effect of genetic ablation studies40. The mammary anlage is laid down close to the nipple and will only start to grow through the mammary fat pad during puberty, giving rise to the mammary epithelial tree. This epithelial anlage can be removed (‘cleared’) by surgery in three-week-old mice, leaving the bulk of the fat pad free of epithelium. Transplantation of primary mammary epithelial cells from a donor mouse results in the outgrowth of these cells into a functional mammary epithelial tree with luminal and myoepithelial cells that can generate milk-secreting alveoli on pregnancy. In our experiments, three weeks after surgery, host females were mated and, in all cases, mammary tissue was isolated for analysis on day 1 of lactation.
Histology, whole-mount staining and immunohistochemistry
For histological analysis, 5-μm sections were cut and stained with haematoxylin and eosin. Whole-mount staining of mammary glands was performed as described8. For immunoperoxidase staining, paraffin-embedded sections were dehydrated and antigenic epitopes were exposed by using a 10 mM citrate buffer and microwaving. Sections were incubated with antibodies against cytokeratin 5, cytokeratin 14, E-cadherin, anti-Ki67 (Novocastra) and anti-active caspase-3 (Cell Signaling) and revealed with peroxidase-conjugated secondary antibodies. Histomorphometric indices (proliferation and apoptosis) were calculated as the number of positive epithelial cells divided by the total number of epithelial cells, with no fewer than 1,000 nuclei for Ki67 stainings and no fewer than 5,000 cells for active caspase-3 staining counted per section.
Western blotting
The human epithelial breast tumour cell line SKBR3 and primary non-transformed mouse mammary epithelial cells were left untreated or stimulated with recombinant murine RANKL41. Adenocarcinomas were isolated from control and mutant mice and total protein lysates were prepared. Western blotting was performed with standard protocols. In brief, blots were blocked for 1 h with 5% BSA in 10 mM Tris-HCl pH 7.5, 150 mM NaCl containing 0.1% Tween 20 (TBST) and incubated overnight with primary antibody at 4 °C (diluted in TBST in accordance with the manufacturer’s protocol). Primary antibodies reactive to mouse RANKL (AF462; R&D), cyclin D1 (catalogue no. Sc-8396; Santa Cruz), β-actin (Sigma), phosphorylated NF-κB (catalogue no. 3033), NF-κB (catalogue no. 4767), phosphorylated IκB-α (catalogue no. 2859), IκB-α (catalogue no. 4814), phosphorylated IKK-α (catalogue no. 2681), IKK-α (catalogue no. 2678), IKK-β (catalogue no. 2678), IKK-γ (catalogue no. 2685), phosphorylated Akt (catalogue no. 3787), Akt (catalogue no. 9272), phosphorylated Erk1/2 (catalogue no. 9101), Erk1/2 (catalogue no. 9102), p38 MAPK (catalogue no. 9212), p53 (catalogue no. 2524), phosphorylated Chk1 (catalogue no. 2348), Chk1 (catalogue no. 2345) (all from Cell Signaling), p38 MAPK (AF869; R&D) and γ-H2AX (Ser 139, catalogue no. 07-164; Millipore) were used. Blots were washed three times in TBST for 30 min, incubated with horseradish peroxidase-conjugated secondary antibodies (1:2,000 dilution; Promega) for 1 h at 20–25 °C, washed three times in TBST for 30 min, and revealed with enhanced chemiluminescence.
Quantitative RT–PCR
Total RNA of tumours was prepared with the RNeasy Mini Kit (Qiagen), in accordance with the manufacturer’s instructions. Total RNA (2 μg) was subjected to quantitative RT–PCR analysis. The following primers were used: β-actin, 5′-GCTCATAGCTCTTCTCCAGGG-3′ (forward) and 5′-CCTGAACCCTAAGGCCAACCG-3′ (reverse); RANKL, 5′-CTGAGGCCCAGCCATTTG-3′ (forward) and 5′-GTTGCTTAACGTCATGTTAGAGATCTTG-3′ (reverse); RANK, 5′-CTTGGACACCTGGAATGAAG-3′ (forward) and 5′-CAGCACTCGCAGTCTGAGTT-3′ (reverse); cyclin D1, 5′-CTGTGCGCCCTCCGTATCTTA-3′ (forward) and 5′-GGCGGCCAGGTTCCACTTGAG-3′ (reverse); p21 (Cdkn1a), 5′-GTGGCCTTGTCGCTGTCTT-3′ (forward) and 5′-GCGCTTGGAGTGATAGAAATCTG-3′ (reverse); tRANKL forward, 5′-GCGCCGGGCCAGCCGAGACTAC-3′; RANKL1 forward, 5′-GTCCCACACGAGGGTCCGCTGC-3′; RANKL 2 forward, 5′-TGCGCACTCCGGCGTCCCG-3′; RANKL 3 forward, 5′-CCGAGACTACGGCGGATCCTAACAG-3′; RANKL common reverse, 5′-TCAGTCTATGTCCTGAACTTTGAAAGCCCC-3′; Puma, 5′-CCGCCTGATGCCCTCCGCTGTAT-3′ (forward) and 5′-CGGGCCCACTCCTCCTCCTCCAC-3′ (reverse); Noxa, 5′-ACTTTGTCTCCAATCCTCCG-3′ (forward) and 5′-GTGCACCGGACATAACTGTG-3′ (reverse); Bim, 5′-GTTGAACTCGTCTCCGATCC-3′ (forward) and 5′-GCCCCTACCTCCCTACAGAC-3′ (reverse).
DNA damage responses
For measurement of cell-cycle arrest and apoptosis, primary mouse mammary epithelial cells and SKBR3 human breast cancer cells were seeded at a cell density of 105 cells per well in a six-well plate and allowed to grow for 24 h. Cells were then treated with doxorubicin (1 μM) or γ-irradiation (2 Gy) in the absence or presence of recombinant RANKL (1 μg ml−1). Cell-cycle arrest and numbers of dead cells were determined by staining with propidium iodide. To determine in vivo mammary-gland epithelial cell death, control and RANKΔmam littermate females were γ-irradiated with a total dose of 5 Gy. Six hours later, mammary glands were isolated and immunostained for active caspase-3 (Cell Signaling), indicative of apoptosis.
FACS analysis of primary mammary epithelial cells
For FACS analysis of mammary epithelial subpopulations, the following protocol for tissue dissociation was used to generate single-cell suspensions: lymph nodes were removed from both inguinal mammary fat pads; fat pads were then digested for 2.5 h in 2 ml of complete EpiCult medium (EpiCult-B basal medium; catalogue no. 05610; StemCell Technologies) supplemented with EpiCult-B proliferation supplements, 10 ng ml−1 basic fibroblast growth factor (catalogue no. 02634; StemCell Technologies), 10 ng ml−1 epidermal growth factor (catalogue no. 02633; StemCell Technologies), 4 μg ml−1 heparin (catalogue no. 07980; StemCell Technologies), 2.5 ml of FBS (5%) and antibiotics) with 2.5 × collagenase/hyaluronidase (for example, 500 μl of 10 × collagenase plus 1.5 ml of Epicult per mouse) (catalogue no. 07912; StemCell Technologies) in 50-ml Falcon tubes at 37 °C. After vigorous vortex-mixing, pellets were washed with 10 ml of HF medium (Hanks Balanced Salt Solution Modified (catalogue no. 07913; StemCell Technologies) plus 2% FBS). Pellets were then resuspended in 2 ml of prewarmed trypsin-EDTA. After a further wash with 10 ml of HF medium, pellets were resuspended in 2 ml of prewarmed (37 °C) dispase (catalogue no. 07913; StemCell Technologies) supplemented with 200 μl of 1 mg ml−1 DNase I (catalogue no. 07900; StemCell Technologies). After a final wash in HF, cells were counted and prepared for FACS staining. Cells (106) were incubated with the following antibodies: biotin-conjugated anti-CD31 (catalogue no. 553371; BD), which labels endothelial cells, and biotinylated CD45+ and Ter119+ (StemSep murine chimaera cocktail; catalogue no. 13058C; Stem Cell Technologies; 3.5 μl per 100 μl), which labels haematopoietic cells, for 10 min at room temperature. Haematopoietic and endothelial cells were excluded by FACS with streptavidin-conjugated allophycocyanin (catalogue no. 554067; BD). Staining with anti-CD49f (catalogue no. 551129; BD) and CD24 (catalogue no. 553261; BD) was used to identify the mammary-stem-cell population as described previously37,38.
Cancer stem-cell assays
Self-renewal of mammary cancer stem cells (TICs) was assayed with a mammosphere assay as described previously28,39. In brief, similar-sized tumours (1 cm3 volume) were minced and digested in complete EpiCult medium with 2.5 × collagenase/hyaluronidase (catalogue no. 07912; Stem Cell Technologies). Cells (2 × 105) were then cultured in serum-free EpiCult medium supplemented with B27 (Invitrogen), 20 ng ml−1 epidermal growth factor (Protech) and 20 ng ml−1 basic fibroblast growth factor (Sigma), using six-well ultra-low-attachment plates (Corning Costar). The primary mammospheres, which formed over 7 days, were collected by gentle centrifugation (60g), digested into single-cell suspensions with trypsin (0.05%, 10 min), and assayed for their ability to form secondary mammospheres as above.
Anchorage-independent growth
The ability of cells to grow in soft agar was assayed as described previously24. In brief, DNA-grade agarose (1% in DMEM) was used as the bottom layer (2 ml in six-well plates) and 2 × 104 SKBR3 cells were seeded in 1.5 ml of agarose (0.3% in DMEM). Cells were overlaid with 1.5 ml of DMEM supplemented with 10% FCS and cultured for 24 days.
Statistics
All values in the paper are given as means ± s.e.m. Comparisons between groups were made with Student’s t-test. For the Kaplan–Meier analysis of tumour onset, a log-rank test was performed. P < 0.05 was accepted as statistically significant. In addition to the log-rank test a post-hoc power analysis was performed (PS Power and Sample Size Calculations, http://biostat.mc.vanderbilt.edu/PowerSampleSize) to calculate the probability of correctly rejecting the null hypothesis of equal tumour onset times given the number of experimental animals. For the study involving the RANKΔmam animals, the null hypothesis can be rejected with a probability (power) of 0.933 and for the IKK-αΔmam animals with a probability of 0.766. The type I error probability associated with this test of this null hypothesis is 0.05.
References
Jemal, A. et al. Cancer statistics, 2009. CA Cancer J. Clin. 59, 225–249 (2009)
Rossouw, J. E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. J. Am. Med. Assoc. 288, 321–333 (2002)
Beral, V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362, 419–427 (2003)
Kong, Y. Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999)
Dougall, W. C. et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 13, 2412–2424 (1999)
Rossi, S. W. et al. RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J. Exp. Med. 204, 1267–1272 (2007)
Hanada, R. et al. Central control of fever and female body temperature by RANKL/RANK. Nature 462, 505–509 (2009)
Fata, J. E. et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 103, 41–50 (2000)
Jones, D. H. et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature 440, 692–696 (2006)
Morony, S. et al. Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res. 61, 4432–4436 (2001)
Cummings, S. R. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361, 756–765 (2009)
Beleut, M. et al. Two distinct mechanisms underlie progesterone-induced proliferation in the mammary gland. Proc. Natl Acad. Sci. USA 107, 2989–2994 (2010)
Pike, M. C. et al. Estrogen–progestin replacement therapy and endometrial cancer. J. Natl. Cancer Inst. 89, 1110–1116 (1997)
el-Mahgoub, S., Karim, M. & Ammar, R. Long-term use of depot medroxy progesterone acetate as a contraceptive. Acta Obstet. Gynecol. Scand. 51, 251–255 (1972)
Hofseth, L. J. et al. Hormone replacement therapy with estrogen or estrogen plus medroxyprogesterone acetate is associated with increased epithelial proliferation in the normal postmenopausal breast. J. Clin. Endocrinol. Metab. 84, 4559–4565 (1999)
Wada, T., Nakashima, T., Hiroshi, N. & Penninger, J. M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 12, 17–25 (2006)
Srivastava, S. et al. Receptor activator of NF-κB ligand induction via Jak2 and Stat5a in mammary epithelial cells. J. Biol. Chem. 278, 46171–46178 (2003)
Aldaz, C. M., Liao, Q. Y., LaBate, M. & Johnston, D. A. Medroxyprogesterone acetate accelerates the development and increases the incidence of mouse mammary tumors induced by dimethylbenzanthracene. Carcinogenesis 17, 2069–2072 (1996)
Asselin-Labat, M. L. et al. Control of mammary stem cell function by steroid hormone signalling. Nature 465, 798–802 (2010)
Joshi, P. A. et al. Progesterone induces adult mammary stem cell expansion. Nature 465, 803–807 (2010)
Cao, Y. et al. IKKα provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107, 763–775 (2001)
Wright, A. et al. Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD. Dev. Cell 13, 705–716 (2007)
Kim, N. S. et al. Receptor activator of NF-κB ligand regulates the proliferation of mammary epithelial cells via Id2. Mol. Cell. Biol. 26, 1002–1013 (2006)
Freedman, V. H. & Shin, S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell 3, 355–359 (1974)
Lee, J. et al. Id helix-loop-helix proteins negatively regulate TRANCE-mediated osteoclast differentiation. Blood 107, 2686–2693 (2006)
Ewan, K. B. et al. Transforming growth factor-β1 mediates cellular response to DNA damage in situ. Cancer Res. 62, 5627–5631 (2002)
Pece, S. et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 140, 62–73 (2010)
Cao, Y., Luo, J. L. & Karin, M. IκB kinase α kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc. Natl Acad. Sci. USA 104, 15852–15857 (2007)
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000)
McTiernan, A. et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: Women’s Health Initiative randomized trial. J. Natl. Cancer Inst. 97, 1366–1376 (2005)
Wagner, K. U. et al. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 10, 545–553 (2001)
Boggio, K. et al. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J. Exp. Med. 188, 589–596 (1998)
Kuhn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995)
Tarutani, M. et al. Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proc. Natl Acad. Sci. USA 94, 7400–7405 (1997)
Gareus, R. et al. Normal epidermal differentiation but impaired skin-barrier formation upon keratinocyte-restricted IKK1 ablation. Nature Cell Biol. 9, 461–469 (2007)
Aliprantis, A. O. et al. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J. Clin. Invest. 118, 3775–3789 (2008)
Stingl, J. et al. Purification and unique properties of mammary epithelial stem cells. Nature 439, 993–997 (2006)
Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 (2006)
Dontu, G. et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17, 1253–1270 (2003)
Robinson, G. W. & Hennighausen, L. Inhibins and activins regulate mammary epithelial cell differentiation through mesenchymal-epithelial interactions. Development 124, 2701–2708 (1997)
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998)
Acknowledgements
We thank all members of our laboratories for helpful discussions; G. Forni for providing NeuT transgenic mice. A.O.A. is a recipient of a Career Award for Medical Scientists from the Burroughs Wellcome Fund. L.G. is supported by National Institutes of Health (NIH) grant HD055601. G.S’s work is supported by the German Research Council (Deutsche Forschungsgemeinschaft (DFG): FOR643, SFB641 and SPP1468), by the Interdisciplinary Center for Clinical Sciences Erlangen and by the focus programme SPP1468 (Immunobone) of the DFG and the Masterswitch project of the European Union (EU). C.J.O. is supported by the National Health and Medical Research Council of Australia, the Australian Cancer Research Fund and the Cancer Institute New South Wales. D.S. is supported by the EU InflaCare network. M.W. is supported by a grant from the University College London (UCL) Hospital/UCL Comprehensive Biomedical Research Centre project no. 152, and part of this work was undertaken at UCL Hospital/UCL, which received a proportion of its funding from the Department of Health National Institute for Health Research Biomedical Research Centres funding scheme. J.M.P. is supported by grants from Institute of Molecular Biotechnology, the Austrian Ministry of Sciences, the Austrian Academy of Sciences, GEN-AU (AustroMouse), an EU Marie Curie Excellence Grant, and a European Research Council Advanced Grant. Special thanks go to Limin Zhang, who provided a first idea for this manuscript and died much too early of breast cancer.
Author information
Authors and Affiliations
Contributions
D.S. performed most experiments together with A.L. (NeuT experiments) and V.S. (experiments with MPA and DMBA). A.L. generated the RANKfloxed mice. L.K. analysed the tumour section as an expert pathologist. R.H. helped with hormone treatment of mice. P.A.J. and R.K. helped with FACS analysis of mammary-stem-cell populations. A.P. helped in gene set enrichments. H.L. and C.J.O. performed the experiments on prolactin receptor mutant mice. L.G., with A.A. and M.P., provided essential mouse strains and input to the paper. G.S. and M.W. helped in human experiments and read the manuscript critically. J.M.P. coordinated the project, wrote the manuscript, and together with D.S. designed the experiments.
Corresponding author
Ethics declarations
Competing interests
J.M.P. declares that IMBA, his host institute, is planning to submit a patent on blocking RANKL/RANK for future treatment/prevention of breast cancer, and that J.M.P. owns shares in Amgen, a company that developed RANKL blocking antibodies. No other competing financial interest is known.
Supplementary information
Supplementary Information
This file contains Supplementary Text containing information on Rank detection in tumors and Cre effects and Gene expression profiling. The file also contains Supplementary Figures 1-16 with legends and additional references. (PDF 3481 kb)
Rights and permissions
About this article
Cite this article
Schramek, D., Leibbrandt, A., Sigl, V. et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468, 98–102 (2010). https://doi.org/10.1038/nature09387
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09387
This article is cited by
-
Single-cell transcriptomics provide insight into metastasis-related subsets of breast cancer
Breast Cancer Research (2023)
-
Aberrant accumulation of NIK promotes tumor growth by dysregulating translation and post-translational modifications in breast cancer
Cancer Cell International (2023)
-
Progesterone from ovulatory menstrual cycles is an important cause of breast cancer
Breast Cancer Research (2023)
-
Postdiagnosis circulating osteoprotegerin and TRAIL concentrations and survival and recurrence after a breast cancer diagnosis: results from the MARIE patient cohort
Breast Cancer Research (2023)
-
Luminal Rank loss decreases cell fitness leading to basal cell bipotency in parous mammary glands
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.