Abstract
The accumulation of species-specific enemies around adults is hypothesized to maintain plant diversity by limiting the recruitment of conspecific seedlings relative to heterospecific seedlings1,2,3,4,5,6. Although previous studies in forested ecosystems have documented patterns consistent with the process of negative feedback7,8,9,10,11,12,13,14,15,16, these studies are unable to address which classes of enemies (for example, pathogens, invertebrates, mammals) exhibit species-specific effects strong enough to generate negative feedback17, and whether negative feedback at the level of the individual tree is sufficient to influence community-wide forest composition. Here we use fully reciprocal shade-house and field experiments to test whether the performance of conspecific tree seedlings (relative to heterospecific seedlings) is reduced when grown in the presence of enemies associated with adult trees. Both experiments provide strong evidence for negative plant–soil feedback mediated by soil biota. In contrast, above-ground enemies (mammals, foliar herbivores and foliar pathogens) contributed little to negative feedback observed in the field. In both experiments, we found that tree species that showed stronger negative feedback were less common as adults in the forest community, indicating that susceptibility to soil biota may determine species relative abundance in these tropical forests. Finally, our simulation models confirm that the strength of local negative feedback that we measured is sufficient to produce the observed community-wide patterns in tree-species relative abundance. Our findings indicate that plant–soil feedback is an important mechanism that can maintain species diversity and explain patterns of tree-species relative abundance in tropical forests.
Similar content being viewed by others
Main
Negative feedbacks occur when detrimental effects of enemies that accumulate in the vicinity of a given adult are expressed more strongly on conspecific relative to heterospecific juveniles. As a result, enemy-mediated reduction of growth and survival of conspecific juveniles near a given adult can provide a localized recruitment advantage for juveniles of other species1,2,3. This process can maintain species richness by preventing any one species from dominating the plant community18,19,20.
In forests, the strongest evidence that negative feedback processes influence plant species composition comes from demographic analyses of spatial and temporal patterns of tree growth and survival. These demographic studies often reveal that seedlings and saplings perform more poorly when in high densities or near conspecific adults7,8,9,10,11,12. Such patterns of density and distance dependence are expected to emerge if the process of enemy-mediated negative feedback is operating in the plant community. Demographic analyses, however, are not able to identify the principal classes of enemies (for example, pathogens, invertebrates, mammals) that drive negative feedbacks, nor are they able to distinguish between enemy-mediated feedback and other possible mechanisms that could lead to similar demographic patterns, such as higher intraspecific competition for abiotic resources near parent trees.
Experimental studies in both temperate and tropical forests have attempted to demonstrate the process of negative feedback and to identify the causal agents. These studies often find that detrimental effects of enemies on seed or seedlings are greater near than away from conspecific trees13,14,15,16. However, with few exceptions21, these studies restrict their analyses to within single tree species, and thus fail to examine whether the effects of enemies are species-specific, which is an essential requirement to provide a recruitment advantage to heterospecific seedlings. Instead, experimental studies that examine performance of conspecific relative to heterospecific juveniles near a host tree are necessary17. Furthermore, simulation models are needed to determine whether empirically based estimates of negative feedback occurring at the local scale of the tree are sufficient to influence community-wide patterns in species diversity and relative abundances.
We first conducted a shade-house experiment designed to assess the importance of soil biota (for example, fungi, bacteria, fauna) in generating negative feedback, while controlling for nutrients and light. We chose six shade-tolerant tree species, the adult relative abundances of which in the 50-ha plot on Barro Colorado Island (BCI) ranged over roughly two orders of magnitude, which allowed us to examine whether variation in the strength of feedback among species was correlated with their adult abundance. We filled all pots with an identical mixture (3:1) of sterilized field soil and sand. To each pot, we added a single seedling along with a small quantity (6% total volume) of either live or sterilized soil inoculum collected from under either conspecific or heterospecific adult trees in a fully reciprocal design. This experimental design allowed us to control for abiotic soil effects, while introducing soil biota. We measured total seedling biomass after 5 months.
We found strong evidence for negative plant–soil feedback based on growth when averaged across all species, with four of the six being significant (Fig. 1a). For these four species, seedling growth was reduced relative to heterospecific seedlings when grown with conspecific versus heterospecific inoculum (see Supplementary Fig. 1). Overall seedling growth (averaged across species) did not differ across sterilized inocula from the different adult species; however, growth did differ significantly across different sources of inocula containing live biota (Fig. 1b). This finding confirms that differences in seedling response were due to differences in live soil biota and not due to differences in abiotic properties of the soil. Furthermore, the strength of negative feedback was correlated with the relative abundance of those adult trees found on the BCI 50-ha plot (P = 0.058). Tree species showing strong negative feedback were less common as adults than were species exhibiting weaker or no significant negative feedback (Fig. 1c).
a, Variation in the strength of negative feedback mediated by soil biota among the six seedling species. Ba, Brosimum alicastrum; Bp, Beilschmiedia pendula; En, Eugenia nesiotica; Lp, Lacmellea panamensis; Tp, Tetragastris panamensis; Vs, Virola surinamensis. Bars indicate standard errors, and means that differ from zero are indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001). Number of seedlings analysed = 349. b, Seedling response (averaged across seedling species) varied across live inocula (ANCOVA: F5,408 = 5.31, P < 0.0001) but not across sterile inocula (ANCOVA: F5,408 = 0.15, P = 0.981). Bars indicate standard errors. c, Tree species that exhibited stronger negative feedback were less common as adults in the BCI 50-ha plot. DBH, diameter at breast height.
We then conducted a reciprocal field experiment in a mainland forest (Gigante Peninsula, Panama) located adjacent to BCI to determine whether patterns of negative plant–soil feedback observed in the shade-house experiment were also found in the forest in the presence of above-ground enemies and other potentially confounding processes. We selected five tree species that differed in adult relative abundance (three of which were used in the shade-house experiment). We grew seedlings in sterilized soil for 1 month and then transplanted them into plots containing all five species in July 2008. A single mixed-species seedling plot was established under each replicate adult tree of each species. We measured growth and survival at the end of the first wet season (January 2009) and survival at both the end of the first dry season (May 2009) and after 16 months, near the end of the second wet season (November 2009).
We found that of the 1,270 seedlings planted into the forest, 945 (74%) survived after 6 months in the forest. All five species exhibited significant negative feedback based on growth of surviving seedlings (Fig. 2a). Consistent with the shade-house experiment, tree species exhibiting strong negative feedback were less common as adults in this mainland forest than those species exhibiting weaker negative feedback (Fig. 2b). Foliar enemies contributed little to the strength of feedback. Leaf damage caused by insect herbivores and foliar pathogens explained an average of only 14% of the overall strength of negative feedback (averaged across all species), with the maximum contribution (28%) occurring in Beilschmiedia pendula due to foliar fungi (Fig. 2c and Supplementary Fig. 2). These findings, combined with the strong effect of soil biota and no effect of nutrients on feedback in the shade-house experiment, indicate that below-ground biota contributed to the majority of growth-based negative feedback measured in the forest (see Supplementary Discussion).
a, Variation in the strength of negative feedback among the five seedling species. Species abbreviations are the same as those in Fig. 1, except for: Aa, Apeiba aspera; Sa, Simarouba amara. Bars indicate standard errors, and means that differ from zero are indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001). Number of seedlings analysed = 945. b, Tree species that exhibited stronger negative feedback were less common as adults in the forest of the Gigante Peninsula than those species exhibiting weaker negative feedback. c, Proportional contribution of foliar insect herbivory, foliar pathogens and other causes to observed patterns of negative feedback.
Mortality during the first 6 months was primarily due to above-ground enemies (uprooting by vertebrates or clipping of stems by vertebrates or insects) and occurred shortly after we transplanted the seedlings. Seedling death caused by these above-ground enemies did not lead to mortality-based feedback when measured in January 2009. However, in a subsequent mortality census conducted in May 2009, faster growing seedlings (as measured in January 2009) had a higher probability of surviving the first dry season than did slower growing seedlings (Supplementary Table 2). By November 2009, estimates of feedback through mortality became increasingly negative (Supplementary Table 3), demonstrating that growth differences among seedlings emerge quickly and develop into mortality-based negative feedback over longer periods of time, as slower growing seedlings are increasingly likely to die.
Analytical models and simulations indicate that negative feedback can maintain plant diversity18,19,20; however, theory on the expected response in tree relative abundance to variation in feedback strength is lacking. In addition, it has been argued that localized processes such as negative feedback may not be sufficient to influence community-wide patterns in tree composition22. We addressed these issues by simulating community dynamics using a stochastic spatially explicit cellular automata model. We found that simulations that included the strength of plant–soil feedback between species pairs measured in our experiments generated community-wide species abundances of similar rank order as those found on BCI and the mainland forest (Fig. 3a, b). Moreover, this pattern holds for simulations of more species-rich communities (Fig. 3c). The correlation between abundance and average feedback was robust when we relaxed the assumption that species had equivalent growth and mortality rates (t = 17.19, P < 0.0001; see Supplementary Methods). When we included variation in species-specific life history traits, a single highly competitive species dominated the simulations in the absence of feedback. Species coexistence occurred only when we also included the empirically measured feedback responses. These simulations demonstrate that the relationship between feedback and abundance is expected only when negative plant–soil feedback is the major force driving plant species coexistence.
a–c, Species abundance generated using simulations including shade-house plant response data (a), field-collected plant response data (b) and randomly generated feedback data (c). All simulations demonstrate that stronger negative feedback leads to lower species abundance. Each circle falling at the same location of the x axis in panels a and b indicates simulated abundance for each of 10 runs. Regression lines per run are plotted in panel c.
Our study is consistent with other findings that soil biota (for example, soil-borne fungi, bacteria, fauna) mediate negative plant–soil feedback in temperate grasslands3,4,23,24,25,26. In a greenhouse experiment, rare temperate grassland species exhibited stronger negative plant–soil feedback due to soil pathogens than more common plant species4. Notably, our field experiment suggests that the correlation between the strength of negative feedback among species and their relative abundance occurs even in the presence of plant competition and other naturally occurring processes in tropical forests. Furthermore, this relationship does not seem to be restricted to just those tree species that we examined. A recent, demographic analysis found a positive correlation between patterns of density-dependent seedling mortality and abundance when 180 tree species on BCI were examined27. Our simulations confirm theoretically that variation in the strength of plant–soil feedback can drive this relationship.
For decades, resource partitioning, above-ground herbivory6 and neutral processes28 have received considerable attention as mechanisms for the maintenance of plant species diversity. However, much of this work has overlooked the effects of soil biota, particularly in species-rich tropical forests. Soil communities are characterized by a great diversity of microbes and fauna26,29, but the extent to which these organisms contribute to the functioning of plant communities is only now beginning to be discovered. By using fully reciprocal experiments, we were able to demonstrate that species-specific interactions between tropical trees and their soil biota are sufficiently strong to maintain tree diversity through negative feedback. Self-limiting processes such as negative plant–soil feedback have been assumed previously to occur more strongly in tree species of high abundance30. However, empirically we found the opposite result: more abundant tree species exhibited the weakest negative feedback. Our simulations reinforce the conclusion that trees are abundant because they are less susceptible to the detrimental effects of their associated soil communities than are rarer tree species. Thus, localized negative plant–soil feedback occurring between plants and below-ground organisms may be a general mechanism for the maintenance of plant species diversity and patterns of relative abundance across ecosystems ranging from temperate grasslands to tropical forests.
Methods Summary
Study species
We selected shade-tolerant tree species from different families that produced sufficient amounts of seeds at the onset of each experiment. We used Beilschmiedia pendula, Brosimum alicastrum and Lacmellea panamensis in both experiments; Eugenia nesiotica, Tetragastris panamensis and Virola surinamensis in the shade-house experiment; and Apeiba aspera and Simarouba amara in the field experiment. We were unable to use identical species sets for each experiment because seed availability varied between the two years in which each experiment was conducted. For each experiment, we collected seeds of all species from their respective forests (shade-house experiment: Barro Colorado Island; field experiment: Gigante Peninsula, Panama), surface sterilized the seeds, and germinated them in sterile soil (see Methods).
Feedback measure
For each experiment, feedback was measured using a priori contrasts within the ‘seedling species × soil-biota source’ interaction term in our mixed-model analysis of covariance (ANCOVA) tests (see Methods). These contrasts isolated the strength and direction of the interaction between seedling species and adult biota source for each possible species pair (that is, pairwise feedbacks18). The strength of average feedback per species was determined by averaging all pairwise feedbacks involving that species18 (see Supplementary Fig. 1).
Stochastic cellular automata simulation
Initially, all cells were occupied and each species was equally represented. A cell was then chosen at random and the species identity was reassigned based on the pairwise plant–soil responses measured in each experiment. Simulations in Fig. 3 assumed species equivalence in growth and mortality. This assumption was then relaxed for subsequent runs of the model (see Methods and Supplementary Equations). The abundance of each species after 20 million replacements was recorded and the correlation with the average strength of feedback per species was tested.
Online Methods
Reciprocal shade-house experiment
We collected seeds from three adults of Beilschmiedia pendula, Brosimum alicastrum, Eugenia nesiotica, Lacmellea panamensis, Tetragastris panamensis and Virola surinamensis located near the 50-ha plot on Barro Colorado Island (BCI). For each adult, we collected and homogenized soil samples from three locations 2 m away from the base of the tree to be used as inoculum. To separate the effects of soil biota from that of potential variation in abiotic properties, we filled all 4-l pots with an identical steam-pasteurized 3:1 sand–field soil mixture. To each pot, we also added a small quantity of live soil inoculum (6% total soil volume) collected from one of the six target species. We planted a single one-month-old seedling of each tree species into pots containing their own live inoculum (conspecific combinations) and pots containing inoculum from each of the five other species (heterospecific combinations). For each tree species, we replicated the conspecific plant–biota combination fifteen times and each heterospecific combination eight times. To confirm that our dilution technique adequately controlled for potential variation in abiotic properties introduced by the small volume of soil inoculum, we assessed seedling growth in the same plant–inoculum combinations, but using sterilized inoculum. Each plant–sterile inoculum combination was replicated twice. We divided all treatment combinations equally across four shade-houses, which were included in the analysis as blocks. Seedlings were well watered and allowed to grow for 5 months, after which seedlings were harvested and total dry weight was determined.
We used mixed-model ANCOVA to examine the main effects of seedling species and soil biota source and their interaction on log-transformed seedling biomass using the SAS procedure PROC MIXED. In this model, we included seedling species and block (and all interactions with block) as random effects, with log-transformed initial biomass as a covariate. We estimated initial biomass per species using regression equations obtained from extra harvested seedlings at the onset of the experiment, where the product of leaf area and stem height was regressed with total seedling dry weights. Within the ‘seedlings species × soil-biota source’ interaction, we used a priori contrasts that isolated the strength and direction of the interaction between seedling species and adult biota source for each possible species pair (that is, pairwise feedbacks18). These contrasts compared the relative growth response of seedlings when associated with soil biota from their own adults versus from under heterospecific adults, relative to how heterospecific seedlings responded across these same soil biota sources (see Supplementary Fig. 1).
Reciprocal field experiment
In July 2008, we transplanted ten seedlings of the same species as the adult (conspecifics) and five seedlings of each of the heterospecific species (30 seedlings total) into a single 1 × 0.8 m grid ∼2.5 m from the base of each adult. Seedlings were randomized and planted 20 cm apart. Ten adult trees of Apeiba aspera, Brosimum alicastrum and Lacmellea panamensis, nine of Simarouba amara and four of Beilschmiedia pendula were haphazardly located in the forest of the mainland Gigante Peninsula, adjacent to BCI. We monitored seedling survival and estimated levels of visible damage (for example, insect herbivory, stem clipping, foliar pathogen infection) biweekly for the first four months, and monthly until the final census. In January 2009, we measured stem height and leaf lengths and widths, and estimated total above-ground biomass using regression equations. These equations were obtained per species by regressing the product of the growth measurements with total above-ground biomass of extra harvested seedlings (4 of 5 species: r2 > 0.91; B. alicastrum: r2 = 0.84). Initial seedling above-ground biomass was obtained in the same manner. The percentage of herbivory or foliar pathogen damage was estimated per leaf for each surviving seedling. We also assessed seedling survival in May 2009 and November 2009.
We analysed survival after 6 months using the SAS procedure PROC GLIMMIX for binomial distributions and log-transformed above-ground biomass using PROC MIXED. Each model included seedling and adult species and their interaction as main effects, and log- transformed initial above-ground biomass (per seedling) as a covariate. The growth model also included number of days between the initial and final census (per seedling) as a covariate. We included ‘site × adult species’ and ‘site × adult species × seedling species’ as random effects, with site defined as a single adult tree (43 ‘sites’ in total). We determined the average feedback per species using methods identical to the shade-house experiment. To investigate the contribution of leaf herbivory and foliar fungal damage to the strength of feedback, we computed the per cent decrease in strength of feedback per species when each damage type was included as a covariate in two additional growth models (see Supplementary Table 2 and Supplementary Fig. 2).
Simulation
We used stochastic spatially explicit cellular automata computer simulations. Each cell on a 300 × 300 torus grid was randomly assigned a species identity. The initial grid contained an equal number of cells per species. Focal cells were then chosen at random and replaced. After 20 million replacements, we examined the abundance of cells representing each species. For all simulations, seeds of each species were assumed to disperse evenly over their 25 surrounding cells. The identity of the new occupant of a replaced focal cell was determined by the establishment probability of each species occurring within the local neighbourhood (25 surrounding cells) of the focal cell. Establishment probabilities were determined by the species-specific response to soil biotic compositions created by both the species previously occupying the focal cell and the suite of species occurring immediately adjacent to the focal cell (surrounding 8 cells). The strength of this response was scaled so that it would be highest immediately adjacent to an adult and taper in strength with increasing distance (see Supplementary Equations). We parameterized two separate simulations where plant response to soil biota (that is, establishment probability) was based on pairwise growth responses measured in either the field or the shade-house experiment. In addition, we simulated a community containing 15 species by assigning the conspecific plant response as a value between 0.1 and 0.2 and the base of the heterospecific pairwise plant response as a value between 0.2 and 0.6, with the individual heterospecific responses being chosen from a uniform distribution within 0.1 of that base value. In this simulation, a new random establishment matrix was generated for each replication. For simulations described thus far, all cells had an equal probability of being selected for replacement (that is, adult mortality rates were assumed to be equal across species). We ran an additional simulation where we relaxed the assumption of species equivalence in mortality by weighting the probability of replacement by estimates of species-specific difference in tree mortality. We also relaxed the assumption that establishment was determined only by plant response to soil biota by weighting this measure by species-specific seedling growth rates (see Supplementary Equations). For all simulations, we replicated each simulation ten times and averaged the correlation coefficients that examined the relationship between the average strength of feedback and tree abundance. Simulations were run in MATLAB.
References
Janzen, D. H. Herbivores and the number of tree species in tropical forests. Am. Nat. 104, 501–528 (1970)
Connell, J. H. in Dynamics of Populations (eds den Boer, P. J. & Gradwell, G. R.) 298–312 (Center for Agricultural Publication and Documentation, 1971)
Bever, J. D. Feedback between plants and their soil communities in an old field community. Ecology 75, 1965–1977 (1994)
Klironomos, J. N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nature 417, 67–70 (2002)
Kulmatiski, A., Beard, K. H., Stevens, J. R. & Cobbold, S. M. Plant-soil feedback: a meta-analytical review. Ecol. Lett. 11, 980–992 (2008)
Carson, W. P., Anderson, J. T., Leigh, E. G. & Schnitzer, S. A. in Tropical Forest Community Ecology (eds Carson, W. P. & Schnitzer, S. A.) 210–241 (Wiley-Blackwell, 2008)
Webb, C. O. & Peart, D. R. Seedling density dependence promotes coexistence of Bornean rain forest trees. Ecology 80, 2006–2017 (1999)
Harms, K. E., Wright, S. J., Calderón, O., Hernández, A. & Herre, A. E. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature 404, 493–495 (2000)
Lambers, H. R. L., Clark, J. S. & Beckage, B. Density-dependent mortality and the latitude gradient in species diversity. Nature 417, 732–735 (2002)
Peters, H. A. Neighbour-regulated mortality: the influence of positive and negative density dependence on tree populations in species-rich tropical forests. Ecol. Lett. 6, 757–765 (2003)
Wills, C. et al. Nonrandom processes maintain diversity in tropical forests. Science 311, 527–531 (2006)
Comita, L. S. & Hubbell, S. P. Local neighborhood and species’ shade tolerance influence survival in a diverse seedling bank. Ecology 90, 328–334 (2009)
Packer, A. & Clay, K. Soil pathogens and spatial patterns of seedling mortality in a temperate tree. Nature 404, 278–281 (2000)
Augspurger, C. K. & Kelly, C. K. Pathogen mortality of tropical tree seedlings: experimental studies of the effects of dispersal distance, seedling density, and light conditions. Oecologia 61, 211–217 (1984)
Hood, L. A., Swaine, M. D. & Mason, P. A. The influence of spatial patterns of damping-off disease and arbuscular mycorrhizal colonization on tree seedling establishment in Ghanaian tropical forest soil. J. Ecol. 92, 816–823 (2004)
Bell, T., Freckleton, R. P. & Lewis, O. T. Plant pathogens drive density-dependent seedling mortality in a tropical tree. Ecol. Lett. 9, 569–574 (2006)
Bever, J. D., Kristi, M. W. & Antonovics, J. Incorporating the soil community into plant population dynamics: the utility of the feedback approach. J. Ecol. 85, 561–573 (1997)
Bever, J. D. Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol. 157, 465–473 (2003)
Adler, R. A. & Muller-Landau, H. C. When do localized natural enemies increase species richness? Ecol. Lett. 8, 438–447 (2005)
Petermann, J. S., Fergus, A. J. F., Turnbull, L. A. & Schmid, B. Janzen-Connell effects are widespread and strong enough to maintain diversity in grasslands. Ecology 89, 2399–2406 (2008)
McCarthy-Neumann, S. & Kobe, R. K. Conspecific plant-soil feedbacks reduce survivorship and growth of tropical tree seedlings. J. Ecol. 98, 396–407 (2010)
Hubbell, S. P., Ahumada, J. A., Condit, R. & Foster, R. B. Local neighborhood effects on long-term survival of individual trees in a neotropical forest. Ecol. Res. 16, 859–875 (2001)
Mills, K. M. & Bever, J. D. Maintenance of diversity within plant communities: soil pathogens as agents of negative feedback. Ecology 79, 1595–1601 (1998)
Kardol, P., Cornips, N. J., van Kempen, M. M. L., Bakx-Shotman, J. M. T. & van der Putten, W. H. Microbe-mediated plant-soil feedback causes historical contingency effects in plant community assembly. Ecol. Monogr. 77, 147–162 (2007)
Bever, J. D. Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. Proc. R. Soc. Lond. B 269, 2595–2601 (2002)
De Deyn, G. B. et al. Soil invertebrate fauna enhances grassland succession and diversity. Nature 442, 711–713 (2003)
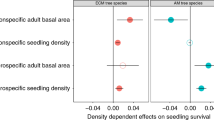
Comita, L. S., Muller-Landau, H. C., Aguilar, S. & Hubbell, S. P. Asymmetric density dependence shapes species abundances in a tropical tree community. Science 10.1126/science.1190772 (in the press)
Hubbell, S. P. The Unified Neutral Theory of Biodiversity and Biogeography (Princeton Univ. Press, 2001)
Roesch, L. F. W. et al. Pyrosequencing enumerates and contrasts soil microbial diversity. ISME J. 1, 283–290 (2007)
Connell, J. H., Tracey, J. G. & Webb, L. J. Compensatory recruitment, growth, and mortality as factors maintaining rain forest tree diversity. Ecol. Monogr. 54, 141–164 (1984)
Acknowledgements
We thank G. Adler, M. Kaspari, E. Leigh, T. Lambert, I. Rubinoff, E. Tanner, M. Tobin, B. Turner, S. Van Bael and N. Wurzburger for providing discussions and comments on the manuscript. R. Kolodziej, K. Meyer, K. McElligott and T. Shirshac provided greenhouse and field assistance. Logistical support was provided by the Smithsonian Tropical Research Institute. The Center of Tropical Forest Science provided BCI tree abundance data published online at https://ctfs.arnarb.harvard.edu/webatlas/datasets/bci/abundance. This study was supported by a Smithsonian Tropical Research Institute (STRI) postdoctoral fellowship to S.A.M., a University of Wisconsin–Milwaukee (UWM) Research Growth Initiative grant to S.A.S., a fellowship from the UWM Research Foundation, and a grant from the National Science Foundation to J.D.B. We thank I. Rubinoff for his support of the STRI Soil Initiative.
Author information
Authors and Affiliations
Contributions
S.A.M. designed and conducted the experiments, analysed the data and wrote the first draft. S.A.S., E.A.H. and J.D.B. provided important revisions. J.D.B. and K.M.L.M. developed the simulation. M.C.V. and E.I.S. provided essential field support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3, Supplementary Figures 1-2 with legends, a Supplementary Discussion, and Supplementary Equations. (PDF 256 kb)
Rights and permissions
About this article
Cite this article
Mangan, S., Schnitzer, S., Herre, E. et al. Negative plant–soil feedback predicts tree-species relative abundance in a tropical forest. Nature 466, 752–755 (2010). https://doi.org/10.1038/nature09273
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09273
This article is cited by
-
Mind the blind spot: lessons from fungal community sequencing in a plant–soil feedback experiment
CABI Agriculture and Bioscience (2023)
-
Modelling how negative plant–soil feedbacks across life stages affect the spatial patterning of trees
Scientific Reports (2023)
-
Plant–soil feedback effects on conspecific and heterospecific successors of annual and perennial Central European grassland plants are correlated
Nature Plants (2023)
-
Negative plant-soil feedbacks disproportionally affect dominant plants, facilitating coexistence in plant communities
npj Biodiversity (2023)
-
Effects of moisture and density-dependent interactions on tropical tree diversity
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.