Abstract
Detection of biomolecules in tissues provides contextual information and the possibility to assess the interaction of different cell types and markers. Routine qualitative assessment of immune- and oligonucleotide-based methods in research and the clinic has been associated with assay variability because of lack of stringent validation and subjective interpretation of results. As a result, the vast majority of in situ assays in clinical usage are nonquantitative and, although useful, often of questionable scientific validity. Here, we revisit the reporters and methods used for single- and multiplexed in situ visualization of protein and RNA. Then we examine methods for the use of quantitative platforms for in situ measurement of protein and mRNA levels. Finally, we discuss the challenges of the transition of these methods to the clinic and their potential role as tools for development of companion diagnostic tests.
Similar content being viewed by others
Main
Biomarkers have been historically considered analytes measured in the blood/sera to determine systemic events. Identification of biomolecules in tissues can have more value than circulating biomarkers as they are accompanied by spatial information, they are closer to the ‘action,’ and they carry contextual information. Often, the context (or its absence) defines the results and validity of the assay (eg, a transcription factor localized to the nucleus). In tissues, the coexistence of multiple cell types in different functional states is a rich source of potential data. This complexity is even more pronounced in biomarker studies of tumor tissues with altered biological composition and frequent aberrant expression of molecules. For example, identification of integral membrane proteins or mRNAs in the cell nucleus, or of transcription factors in the cytoplasm, may carry biological information about function that can be inferred from localization.
In the clinical diagnostic setting, the vast majority of usage of immunohistochemistry (IHC) is not measurement but binary assessment of the contextual information of the biomarker.1 IHC has also been used for measurement. The ability to estimate the level of expression of a given marker within a specific tissue compartment (HER2 in the membrane of breast cancer epithelial cells) has led to assays that have gained FDA approval and to prescription of drugs to subsets of cancer populations that could not be achieved by assays where tissue is ground up or assays where analytes are measured in blood.
Here, we examine the IHC assay and extensions of this assay (quantitative immunofluorescence (QIF)) for measurement of diverse analytes in tissue. We describe the methods for in situ measurement using chromogens or fluorophores and the advantages and disadvantages of each. We also describe methods for quantification of these biomolecules and a vision for translation of these methods to clinical CLIA lab setting.
TISSUE BIOMARKER SIGNAL DETECTION SYSTEMS
Chromogenic Staining
Chromogens are molecules that allow detection of a target using enzyme-based precipitation reactions. They are used in IHC as they allow visualization of the immune complex (and hence the antigen) in the context of tissue architecture. Hematoxylin, the blue component of the hematoxylin and eosin stain, binds to negatively charged molecules (predominantly nucleic acids) and provides a counterstain for the chromogen. Different chromogenic compounds are commercially available in a range of colors.2 The most widely used, 3,3’-diaminobenzidine (DAB), is a highly thermochemically stable polybenzimidazole that provides brown-colored staining.3 The chromogen deposition occurs through a redox reaction4 catalyzed by an enzyme conjugated to an antibody or oligonucleotide detection scaffold.5, 6 This allows direct, bright-field light microscopy assessment of spatial distribution and quantity of a target in counterstained slide preparations.
Optimal chromogenic staining relies on the deposition of a sufficient amount of substrate to block light.7 In the case of DAB, a ‘desirable’ image is produced when the deposition of substrate leads to an absorbance of 1–2 units. This means that 90 to 99% of the light signal is blocked. Although this creates a contrast that is easy to read, it hampers the use of multiple colocalized chromogens on routine assays. Still, different colored chromogens may be used simultaneously to recognize the presence of two different targets and determine their relationship to each other. Chromogens have a dynamic range of nearly one log and are not compatible with in vivo imaging. However, chromogenic-based assays are widely used in biosciences and anatomic pathology because of their ability to localize the antigen in a familiar morphological context, easy interpretation, and simple equipment requirements.
Fluorescent Staining
Fluorescent reporters are widely used as labels in biology and medicine. They are molecules capable of absorption and emission of light at different wavelengths. Absorption of light results in a transition from ground- to excited-electronic state. Then the internal relaxation of the excited state results in radiative decay that emits light (photons), usually at a higher wavelength than the absorption peak.8 Various organic molecules, such as xanthenes, cyanines, and Alexa® dyes,9 are commercially available and encompass a wide excitation/emission spectrum from ∼350 to 800 nm. Advances in nanomaterials have generated new types of inorganic fluorescent molecules with superior photophysical characteristics. Among them, quantum dots10 are luminescent, nanometer-sized superconductor crystals that possess high quantum yield, narrow emission wavelength, and high resistance to photobleaching and chemical degradation. Covalent binding of functional groups11 has allowed their application to immune- or oligonucleotide-based assays using the avidin/streptavidin–biotin reaction. However, their size, hydrophobicity, and specific solvent requirements have limited their use in immunofluorescent applications.
Optical detection of molecules using fluorescence depends on photon emission. Available fluorophores that emit in the visible region of the spectrum possess a broad dynamic range, 2 to 2.5 logs, that makes them good for both visualization and quantification.9 Simultaneous measurement of multiple fluorescent dyes is extensively used for in vitro and in vivo assays, as well as in archival tissues. For appropriate results, the user must select combinations of dyes that have distinct, non-overlapping emission spectra. The limit to the number of dyes used in combination is a function of the emission bandwidth, commonly limiting routine use to 4 or 5 fluorophores synchronously. Postprocessing of the signal to remove overlapping signal or spectral unmixing12 can expand the number of multiplexed fluorophores to 6–8 or more (Richard Levenson, personal communication). New inorganic fluorescent nanomaterials may further increase the number of detectable combinations because of their narrower spectra.
Signal Amplification Systems
Amplification systems aim to increase the ease of detection of the signal by increasing the number of chromogenic or fluorescent molecules associated with each epitope. The first breakthrough involved using enzymatic amplification. By linking an enzyme to the Fc region of an antibody, cyclic enzymatic activity results in multiple deposition events so that a single molecule of enzyme might cleave thousands of molecules of substrate, resulting in at least 3–4 log amplification.13, 14, 15 The most common methods for enzymatic amplification are the peroxidase (horseradish peroxidase (HRP)) and phosphatase (alkaline phosphatase (AP)) reactions.5, 16 Another approach is biotin–avidin method. Here antibodies are biotinylated and each biotin can bind 4 molecules of avidin17 that may be directly linked to a fluorophore or chromogen, or is more commonly linked to an enzyme, thus further amplifying the enzymatic system.18, 19 Another example of additive amplification can be achieved by the use of long-chain polymers conjugated with HRP.20, 21 Incubation with a primary antibody is followed by binding of a secondary antibody conjugated to dextran or similar polymeric backbone that is then conjugated to 100 or more HRPs. Even further signal intensification can be accomplished through the deposition of tyramine compounds. Oxygen free radicals, liberated by peroxidase enzymatic reaction, result in crosslinking of tyramine to nearby proteins. The tyramine that is conjugated to biotin or directly to fluorophore provides another enzymatic amplification step to enhance visualization of antibody–antigen interaction.13 In each case, these amplification systems are typically used to saturation. That is, sufficient substrate is provided such that the amount of conjugated enzyme is the limiting factor for the deposition of signal (chromogen or fluorophore).
Some newer methods involve the use of DNA amplification to increase the signal. Rolling circle amplification (see below)22 uses a ligation reaction to form circular DNA or simply conjugation of circular DNA to a secondary antibody. This is then followed by addition of a short, complementary DNA primer and an enzyme to produce a single-stranded concatameric DNA molecule composed of thousands of copies of the original circles. Fluorophore-labeled oligonucleotide detectors are then used to bind the single-strand sequences. It has largely been used for nucleotide sequence detection, although it has been adapted to immune-based assays.23
Multiplexed Biomarker Detection Assays
Simultaneous interrogation of multiple targets in a single sample (ie, target multiplexing) provides information on tissue distribution, marker colocalization, and synchronous-level quantitation. This approach has been extensively applied on immune-based assays24, 25, 26, 27 and requires a combination of primary antibodies from different species together with species and isotype-specific secondary antibodies or other methods to prevent secondary antibody crossreactivity. The concurrent use of chromogenic reporters requires deposition of different substrates and is limited by the ability to distinguish different colors on routine light microscopy. It is also limited by the physics of the process. That is, if the first chromogen absorbs 95% of the light, only 5% is left for second or subsequent chromogens, limiting the multiplexing capacity. Fluorescent compounds are better suited for this purpose as they use emitted light rather than light absorption. However, fluorophores are limited by the overlapping photon emission spectra.
These problems can be addressed to some extent by either spectral unmixing or cycling of fluorescent substrates. Spectral unmixing7, 12, 28, 29 refers to the pixel-by-pixel determination of the relative contribution of each spectra to the overall signal intensity, providing the means for marker colocalization determination.30 This approach also allows postprocessing of the signal to unmix or subtract fluorophores or chromogens to allow visualization with better signal-to-noise ratio. Postprocessing can also be used to pseudo-color the resulting image to provide a more familiar appearance for fluorescent images.31, 32 Cycling or sequential staining and capturing of fluorophores provides another approach to high-level multiplexing. Gerdes et al33 used sequential fluorescent staining and alkaline quenching, resulting in imaging of up to 61 targets in a single tissue sample. They also show consecutive staining of the same protein for up to 10 cycles. However, this method is limited by the fact that some of the markers showed decreased detection sensitivity as a result of the dye inactivation process, thus limiting linear quantification.34
MEASUREMENT OF CANCER BIOMARKERS
Measurement of Proteins
In the clinical setting, most pathology laboratories perform in situ protein detection using single-marker chromogenic IHC with primary monoclonal antibodies and secondary antibodies conjugated with polymer-based amplification systems. Typically, diverse areas from one slide are evaluated and a trained observer (eg, a pathologist or researcher) renders an integrated categorical estimation of results. This approach has become the pathology standard because of its simplicity, low cost (eg, requires only a traditional light microscope), and preservation of contextual morphological information. Routine examples of in situ protein biomarkers used in the clinical setting include tissue differentiation markers (eg, keratins, vimentin, S100-protein, CD45, CD3, CD20, CD30, TTF-1, CDX2, BRST-2, HMB-45, RCC, HEPAR1, and so on), microorganism-related proteins (eg, viral antigens (hepatitis, HHV-8, CMV, EBV, BKV, and so on), H. pylori, Klebsiella, Pneumocystis, fungal elements, and so on), and specific anticancer therapeutic targets (ERα, PgR, AR, HER2, ALK, ROS1, and so on). In all these cases the presence of the protein of interest is evaluated in strict correlation with the cell type and compartment where it is detected. Typically, the markers are interpreted in a binary manner as present or absent that supports (and/or rules out) a determinate diagnosis. However, the output format and threshold for positivity/negativity is marker specific and subjective.35 Efforts to quantify in situ protein signals have been pursued predominantly for anti-cancer therapeutic targets. In these rare cases (ER, PgR, HER2, Ki67, and may be others at some institutions), semiquantitative scoring systems assess the location, relative intensity, and estimated percentage of positive cells. For the breast cancer markers ERα, PgR, and HER2, guidelines have been written by expert panels in attempts to unify and standardize the IHC approach.25, 36, 37, 38 Although it is the current standard, traditional IHC has been slow to adopt more rigorous quantitative methods.
To date, only two areas of anatomic pathology use systematic immunofluorescence (IF) studies for characterization of specific autoimmune/inflammatory disorders (eg, renal pathology and dermatopathology). This is ironic as IF predated IHC in early efforts to visualize proteins in situ. Fluorescence-based IHC or IF is also widely used in research laboratories39, 40 with single-target and multiplexing approaches, using diverse illumination devices (mercury/xenon light sources, LED illumination systems, and laser beams) and imaging modalities (eg, epifluorescence with optical lenses, pinhole-based confocal microscopy, spinning disc-based confocal imaging, multi-photon imaging, and total internal reflection (TIRF) devices). Comprehensive description of each of these modalities and their most common uses is beyond the scope of this essay and has been described elsewhere.41 In general, the major advantages of IF include its broad dynamic range, capability for multiplexing using different fluorescence channels, amenability for colocalization studies, fluorescence energy transfer protocols (FRET), and signal quantification using digital pixel measurements. These methodologies and others have been extensively used in preclinical mechanistic studies in cell and molecular biology, but surprisingly little in clinical labs in anatomic pathology. This is difficult to understand as increased desire to find mechanisms to match patient subsets to drugs brings increasing demands and complexity to biomarker studies. In fact, the absence of rigorous quantitative tests may in part be a cause for failure of some recent biomarker-driven clinical trials.42
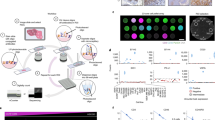
During the past decade, developments allowing more accurate and automated signal quantification of IHC- and/or IF-stained slides have become available including the Inform® software (Caliper/Perkin-Elmer), TissueStudio® (Definiens/Leica), Ariol® (Genetix/Leica Microsystems), VIASTM (Ventana Medical Systems), AQUA® (HistoRx/Genoptix), and ImageScope® (Aperio Technologies/Leica), among others. These platforms use image segmentation and feature extraction-based signal quantification algorithms to measure the signal in selected areas or cells43, 44 In particular, some of these systems (Ariol, ImageScope, and VIAS) have received FDA approval for clinical use in breast cancer.43, 44 Other systems, including Multi-omyx, INform, Definiens, Tissuegnostics, Visopharm, and the AQUA method, use multiplexed IF and can measure targets in defined tissue regions, by feature extraction, more complicated spatially defined compartments, or compartments defined by molecular colocalization with specific proteins to generate objective (eg, human independent) region-specific scores.43, 44 For example (Figure 1), simultaneous visualization of cytokeratin (tumor cells), CD3 (T lymphocytes), CD8 (cytotoxic T cells), CD20 (B lymphocytes), and DAPI signal (all nuclei) allows characterization and quantification of TILs in the tumor and stromal areas.45, 46 Beyond traditional multiplexing, a cycling approach has recently been described where 60–100 protein targets are identified in the same sample.33, 47 The next generation of multiplexing is also at a very early stage. Two groups have reported the ability to multiplex up to 40 (with promises of 100 or more) proteins in FFPE tumor tissues using antibodies labeled with isotopic metals in the lanthanide series followed by detection using secondary ion mass spectrometry.48, 49 These rare earth metals produce a highly distinct signal in mass spectrometry with minimal if any signal overlap, showing the potential to overcome most of the current limitations of multiplexed in situ protein measurement. However, the mass spectrometry-based detection systems are still under development and will require further technological advancements before broad adoption.
Multiplexing targets in FFPE tissues using immunofluorescence. (a) Schematics of the serial multiplexing protocol for simultaneous staining of CD3 (red-colored text), CD8 (green), CD20 (purple), cytokeratin (yellow), and DAPI (blue) in formalin-fixed, paraffin-embedded tissues. The primary antibodies, isotype-specific secondary antibodies, and fluorescence detection system are indicated. (b) Representative low-power microphotographs showing a hematoxylin and eosin-stained preparation of human tonsil (upper left). The same section was stained with the multiplexing TILs protocol indicated in (a) and the fluorescence images in each channel (same magnification) are shown for each marker.
Measurement of RNAs
Until recently, the in situ detection of mRNA using nonradioactive in situ hybridization (ISH) strategies were largely confined to identification of relatively high abundance transcripts, largely for research purposes.50, 51 Similarly, clinical use of in situ RNA detection was limited to identification of highly expressed EBV-associated proteins LMP-1 and EBER using chromogenic ISH to support the diagnosis of some epithelial and lymphoid neoplasms (eg, nasopharingeal carcinoma, endemic Burkitt’s lymphoma, lymphomatoid granulomatosis, posttransplant lymphomas, and so on). More recently, novel in situ hybridization strategies using increased numbers of hybridization probes/per target, in situ target sequence amplification, and novel signal enhancement methods have allowed detection of low-abundance mRNA transcripts in conventional FFPE tissues. Coupling of these methods with sensitive signal measurement/quantification tools has opened new avenues for the use of RNAs as cancer biomarkers.
There are four methodologically unique methods for in situ mRNA detection platforms using fluorescence-based signal detection that have the potential to detect single mRNA molecules. They are: (1) paired probe-based ISH assays (RNAscope® and QuantiGene RNAview®); (2) single-tagged multiple probes ISH (Stellaris® assay); (3) locked nucleic acid-based RNA detection (LNATM probes), and (4) in situ amplification/labeling-based systems (rolling-circle amplification with padlock probes). For information regarding additional RNA ISH protocols and novel DNA ISH methodologies for cancer diagnostics, we refer the readers to comprehensive reviews published elsewhere.50, 52, 53, 54, 55
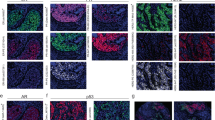
Perhaps the most prominently used method is the paired probes method for mRNA ISH (also known as Z-probes or branched probes), based on the contiguous hybridization of various pairs of 14–20-long RNA oligonucleotides spanning typically an ∼1 kb area. Each probe is designed with a target-specific sequence, a spacer, and a tail sequence that is recognized by the signal amplification HRP- or AP-based system only when serially aligned with its partner probe (and not with potentially nonspecific single-bounded probes).56 The major advantages of this method are the high-level signal amplification, the noise reduction achieved by the paired Z-probe method, and the facilitation of the parallel use of positive and negative control probes (eg, Ubiquitin C or GAPDH as positive controls; and bacterial DapB as negative control) to determine sample integrity and experimental quality. Figure 2 shows an example of PTEN, UbC, and DapB stained in serial TMA sections and quantified using the AQUA method by multiplexing with pancytokeratin stain to define the tumor compartment. The paired probe system is available in two commercial assays (RNAscope and QuantiGene RNAview), providing a vast array of target probes and possibilities for customized probe design. The two commercial platforms share the paired probe design, but may differ in their signal detection method.
Measurement of PTEN mRNA in FFPE tissues using in situ hybridization with the paired probes assay (RNAscope) coupled to quantitative fluorescence. (a) Representative fluorescence microphotographs showing in situ detection of PTEN mRNA (upper left, red fluorescence channel), Ubiquitin C mRNA (UbC, red channel, middle panel), and DapB mRNA (red channel, right panel) in archival breast cancer specimens. The lower panels show the cytokeratin stain in each tissue sample (green fluorescence channel). UbC was used as positive control for the presence of measurable mRNA and bacterial DapB was used as negative control and noise indicator for each sample and in each run. (b) Chart showing the levels of PTEN mRNA (blue columns), UbC mRNA (red columns), and DapB (green columns) in archival FFPE breast cancer samples. Serial sections from a tissue microarray including samples from 238 breast carcinomas (YTMA128) were stained simultaneously for each mRNA target and with cytokeratin protein. The levels of each marker were measured in the tumor compartment using the AQUA technology and are expressed as arbitrary units of fluorescence (y axis). Only spots including available scores for all three mRNA markers are included in the chart.
The single-label probe mRNA ISH approach was first described targeting each transcript of interest with 30–50 short (17–22 nucleotides long), singly fluorescently labeled RNA oligonucleotides.57, 58 This method allowed simultaneously allocation of many fluorescent molecules to each target transcript and was shown to be sensitive, specific, and suitable for FFPE samples. This method also allowed multiplexed target detection using different fluorescence channels. Although earlier studies have used variations on the single probe theme, none are widely published. More recently, a more comprehensive version of this assay became commercially available as the Stellaris RNA FISH and includes predesigned target oligonucleotides with bounded fluorophores as well as an online webtool for personalized probe design. To date, diverse studies have communicated results using this platform in in vitro cell preparations.59, 60, 61, 62, 63, 64, 65, 66 However, and to our knowledge, only two reports (from the same researchers) have used the Stellaris FISH assay to interrogate the association between expression of RIP2 and KIF14 transcripts in human breast cancer specimens.67, 68
The locked nucleic acid (LNA)-based RNA in situ detection is based on oligonucleotide probes made with chemically modified nucleotides that can increase the duplex stability at higher temperatures and increased specificity as compared with conventional RNA probes. The use of anti-digoxigenin HRP-conjugated antibodies after hybridization allows using signal amplification systems to detect the molecules. In particular, digoxigenin double-labeled and relatively short (12–24 nucleotide) LNA probes have been successfully used to detect mRNAs in cells and tissue preparations.69, 70 However, the main use of this technology in human tumors has been to detect and measure microRNAs.71, 72 Using this approach coupled to quantitative fluorescence, we have shown prognostic value of miR-221 in human breast cancer73 and the tumor suppressive role of miR-205 in human melanoma.74 Others have used this method to show expression of small RNAs (microRNAs and lncRNAs) in diverse human tissues and in archival biopsy material from various tumor types including pancreatic, breast, colorectal, nasopharyngeal, and lung carcinomas.75, 76, 77, 78, 79, 80, 81, 82
The padlock probes/rolling-circle amplification system was described nearly a decade ago and was originally used for DNA FISH and genotyping.83, 84, 85 This system has also been used for tissue mRNA visualization.86, 87 Padlock probes are linear oligonucleotides that bind reverse-transcribed cDNA of the mRNA of interest. After hybridization, probes are circularized by high-stringency ligation and the circular DNA padlock probe can act as template for rolling-circle replication steps using DNA polymerase and several amplification steps. The successive amplification generates multiple concatemers including the target sequence and several linker probe sequences. These linker sequences serve then as hybridization sites for fluorescently labeled oligonucleotides that are used to recognize the target. Using initial reverse transcription with LNA primers, this method was successfully used to detect single mRNA transcripts in paraformaldehyde-fixed human and mouse tissues.86 Moreover, the high primer specificity and the high fidelity ligase step also allowed the identification of single-base substitutions of the target transcript. This approach was recently used to identify mRNA mutations and characterize the expression of 39 different transcripts (including 21 targets from the Oncotype DX test) using a novel ligation-based sequencing bar-coding system in fixed frozen sections from human breast tumors.69 This method of in situ mRNA measurements could be performed in cytological imprints and FFPE tissues,88 and an automated platform to analyze this assay has been developed as an ImageJ plugin for signal quantification.88, 89
STANDARDIZATION AND MEASUREMENT IN THE RESEARCH LAB AND IN THE CLIA-CERTIFIED LAB
Quantitative Standardization of Predictive Cancer Biomarkers
Current ASCO/CAP guidelines for the interpretation of estrogen receptor (ER)36 and human epidermal growth factor receptor 2 (HER2, ERBB2)37 consider qualitative, chromogen-based IHC for status determination in breast cancer. Guidelines have been published by the CAP to validate antibodies90 and the FDA has cleared a number of assays for both pathologist-read and semiquantitative analysis of hormone receptors and HER2.91 However, even with protocol-locked robotic stainers and prediluted antibodies, IHC is still subject to considerable variability because of lack of tissue-based standardization92, 93 and subjective pathologist interpretation,94 among other factors. The effect of ‘by-eye’ assay optimization (acceptable to both CAP and the FDA), which is the current standard used to determine the quality of chromogenic staining, is still highly variable. A small study done by placing breast cancer TMAs into the work flow of two separate CLIA labs showed that variability is still present (Figure 3). Although that study was not a rigorous comparison of multiple labs and was limited by the fact that it was done on TMAs, the discordance is concerning and further studies have been proposed. This level of discordance has not been reported in systems read ‘by eye’ but that may represent the inaccuracy of human-based assessment compared with machine assessment. It may also be the result of subtle differences in antibody concentration because, as illustrated by McCabe et al95 and Welsh et al,96 antibody concentration can affect the scoring and the signal-to-noise threshold, thereby potentially changing the apparent expression of a given case. Regardless of the scientific basis, discordance studies are both politically and logistically challenging. To our knowledge, a comprehensive, quantitative assessment of biomarker concordance between multiple institutions has not been done.
Discordance in predictive biomarker assessment between CLIA-certified labs. (a) A TMA with close to 500 spots was put into the daily work flow of two CLIA labs doing estrogen receptor (ER). The spots were then read independently by an author on this work, according to the 2010 ASCO/CAP guidelines (>1% of cells positive) as part of an effort to determine whether discordance was a function of percentage of cells positive for ER. Note that the overall discordance between the labs, with both using FDA-approved methods on automated staining platforms, is 18.7%. (b) Analysis of discordant spots revealed that they are distributed across the range of percentage of positive nuclei. A limitation of this work is that it does not represent whole tissue sections, but rather single TMA spots.
A few studies have examined the effects of antibody concentration and staining conditions on cut-point for estrogen receptor. Using a panel of ER-negative and -positive breast cancer cell lines, Welsh et al96 determined a threshold concentration that separated the cell line groups. By using quantitative IF in the same cells, ER concentration was translated into a continuous score. When these results were compared with conventional assessment of chromogenic IHC, 10–20% of ER-positive cases from two large breast cancer cohorts tested QIF positive/IHC negative. In this case, the decreased dynamic range of chromogenic IHC or perhaps low signal overwhelmed by hematoxylin counterstain did not allow detection of patients with lower levels of ER who would benefit from tamoxifen treatment. Assay/antibody selection also plays a role in predicting outcome. Cheang et al97 showed that selection of SP1, a higher affinity rabbit antibody, showed that the 8% cases that were discordant (positive for SP1and negative for 1D5) showed outcomes similar to concordant positives. Using a similar approach, Welsh et al98 showed an ER-positivity threshold was a function of the antibody tested in two retrospective cohorts using the same two validated antibodies. These studies showed that 7–11% of cases were positive using the antibody clone SP1, but not 1D5. It is notable that both of these antibodies are FDA cleared, suggesting FDA clearance is not currently as standardized and reflective of outcome for biomarkers as it is for therapeutics. It is also notable that when tested ‘by eye’ no difference was seen between the antibodies.99
Quantitative in situ assessment of mRNA is less well studied and used much less in the clinic compared with IHC. Before its mainstream usage, the performance, reproducibility, and interassay variation of the in situ mRNA detection strategies will require careful validation. The relatively lower level of expression of mRNAs compared with proteins and the sensitivity requirements for measuring low-abundance transcripts are still a major concern. In addition (and analogous to detection of proteins), the use of different signal amplification and detection systems and the design of probes targeting transcript regions of variable size could affect interassay reproducibility. Future studies will be required to address these points before common usage of in situ RNA measurements as a clinical tool.
Transition from the Research Lab to the Clinical Lab
Evaluation of tissue biomarkers can reveal their prognostic/predictive value and lead to the development of companion diagnostics. The implementation of such tests in clinical practice as a laboratory-developed test (LDT) requires validation of the test, as outlined in Fitzgibbons et al.90 An LDT is an in vitro diagnostic test that is designed, manufactured, and used within a single laboratory. LDTs can be used to evaluate a wide variety of analytes and can range from relatively simple tests to rather complex assays, such as multiplexed detection of numerous biomarkers. Although LDTs are important to the continued development of optimized diagnostic tests, their widespread use and direct impact on clinical decisions has raised concern in the US Food and Drug Administration.100 If the test is intended to be sold as a kit that can be performed in multiple labs, then the test is an in vitro diagnostic (IVD) and requires FDA clearance to be sold in the United States. Whether the test is an LDT or an FDA-cleared IVD, there is still no guarantee that it will be reimbursed. The Center of Medicare and Medicaid Services (CMS) and other third-party payers make individual decision on reimbursement based on clinical utility. Clinical utility101 should be distinguished from analytic or clinical validity. Clinical utility refers to the actionable value of the test, as determined by high levels of evidence102 that result in changes in patient care as a function of the outcome of the test. The lack of independent review and evidence for clinical utility of LDTs is one the FDA’s main concerns. As CLIA labs may produce and sell LDTs without any proof of clinical validity or utility (they only need to establish analytic validity), they may market and sell tests without proven value to the patient. To address this issue, the FDA has issued a draft guidance on future regulation of devices (including diagnostic tests like LDTs). They outline a timeline to regulation of LDTs to assure that the tests used in the provision of health care are safe and effective.
LDTs and IVDs in Clinical Trials and in the CLIA Lab
Recent advances in immunotherapy have opened new opportunities to patients with advanced-stage solid tumors. Targeting of co-inhibitory molecules, such as programmed death-1 (PD-1) and its ligand PD-L1, have resulted in unprecedented and lasting responses in patients previously treated with diverse standard therapies.103, 104 IHC-based PD-L1 testing has been considered as criteria for inclusion in clinical trials, but its evaluation has not been well defined and assays show wide variability and subjective interpretation. Moreover, the specificity and reproducibility of commercially available antibodies has not been assessed.45, 105, 106 Several ‘positivity’ cut-points have been proposed and used in different tumor elements.103 Although PD-L1 membranous positivity in more than 5% of tumor cells was found to have predictive value in the first reports, some negative cases still exhibited response.104 Other trials have used other criteria for positivity including distinguishing stroma and epithelial staining, and using trial-specific cut-points. Although there is justified fear of depending on LDTs as companion diagnostic tests, it is not clear that specific labeling and FDA-cleared IVD tests will be the solution. Current immune checkpoint trials are using highly variable and drug-specific cut-points, and vendor-specific labs and tests. It is possible that specific drugs may be approved with ‘labeling’ for companion diagnostic assays that have different requirements and different cut-points. This scenario emphasizes the need for standardization in IHC-based testing.
The solution to this problem is not clear. IHC for EGFR failed as a companion diagnostic test in the past for Erbitux.107 In the case of the MET IHC test, it is possible that its subjectivity or limited reproducibility contributed to the recent failure of the MetMab phase 3 trial.42 These mishaps suggest that, in the future, pathologists and oncologists will need to move to objective testing to attempt to take protein measurement to a point where it can be used as a reliable companion diagnostic test. Standardization and measurement are also likely to be important as target molecules such as mRNAs and other small noncoding RNAs enter the predictive biomarker field. One possible solution is the use of QIF. It is capable of objective, multiplexed interrogation of routine FFPE tissues and can accommodate rigorous standardization. Thus, we believe it has the potential to be used to develop next-generation companion diagnostic tests. QIF has been used to predict response to therapy25, 93, 108 and accomplish objective and reproducible assay validation.93 However, resistance to adopt QIF has historically prevented the use of these tests in routine CLIA lab settings. To date, QIF has been introduced in a couple of diagnostics labs (Genoptix and Clarient/GE), but with limited market uptake. It will be interesting to see whether QIF will advance to prominence for tissue-based companion diagnostics, or if other technologies with similar potential for standardization and quantification48, 49, 109 will fill the need for precision tissue-based research and diagnostics.
CONCLUSION
QIF allows objective, in situ interrogation of biomolecules in tissues. This method can be coupled to immune- and oligonucleotide-based assays to detect analytes at the protein and RNA levels. As a recently available LDT in the CLIA lab context, QIF has proven to be a unique tool for assay validation/standardization and investigation of relevant targets for research and clinical purposes. As pathology and oncology move from qualitative to quantitative, and as measurement of biomarkers demands accuracy and precision, new test methods will need to be adopted. It will be interesting to follow these developments over the next few years. One possibility is that IHC will be relegated to a binary qualitative test and other modalities like mRNA by RT-PCR or Nanostring will be used for companion diagnostic testing. However, it is also possible that the drive to quantification will elevate IHC in the form of QIF to bring protein assessment to the quantitative level needed for reproducible companion diagnostic tests.
References
Koh J, Go H, Kim MY et al. A comprehensive immunohistochemistry algorithm for the histological subtyping of small biopsies obtained from non-small cell lung cancers. Histopathology 2014;65:868–878.
van der Loos CM . Chromogens in multiple immunohistochemical staining used for visual assessment and spectral imaging: the colorful future. J Histotechnol 2010;33:31–40.
Graham RC Jr., Karnovsky MJ . The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 1966;14:291–302.
Tubbs RR, Sheibani K, Deodhar SD et al. Enzyme immunohistochemistry: review of technical aspects and diagnostic applications. Cleve Clin Q 1981;48:245–281.
Nakane PK, Pierce GB Jr. . Enzyme-labeled antibodies: preparation and application for the localization of antigens. J Histochem Cytochem 1966;14:929–931.
Speel EJ . Robert Feulgen Prize Lecture 1999. Detection and amplification systems for sensitive, multiple-target DNA and RNA in situ hybridization: looking inside cells with a spectrum of colors. Histochem Cell Biol 1999;112:89–113.
Rimm DL . What brown cannot do for you. Nat Biotechnol 2006;24:914–916.
Nie S, Zare RN . Optical detection of single molecules. Annu Rev Biophys Biomol Struct 1997;26:567–596.
Waggoner A . Fluorescent labels for proteomics and genomics. Curr Opin Chem Biol 2006;10:62–66.
Coto-Garcia AM, Sotelo-Gonzalez E, Fernandez-Arguelles MT et al. Nanoparticles as fluorescent labels for optical imaging and sensing in genomics and proteomics. Anal Bioanal Chem 2011;399:29–42.
Xing Y, Chaudry Q, Shen C et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nat Protoc 2007;2:1152–1165.
Levenson RM . Spectral imaging perspective on cytomics. Cytometry A 2006;69:592–600.
Adams JC . Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem 1992;40:1457–1463.
Hunyady B, Krempels K, Harta G et al. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem 1996;44:1353–1362.
Tsutsumi Y, Serizawa A, Kawai K . Enhanced polymer one-step staining (EPOS) for proliferating cell nuclear antigen (PCNA) and Ki-67 antigen: application to intra-operative frozen diagnosis. Pathol Int 1995;45:108–115.
Mason DY, Sammons R . Alkaline phosphatase and peroxidase for double immunoenzymatic labelling of cellular constituents. J Clin Pathol 1978;31:454–460.
Guesdon JL, Ternynck T, Avrameas S . The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem 1979;27:1131–1139.
Cordell JL, Falini B, Erber WN et al. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 1984;32:219–229.
Sternberger LA, Hardy PH Jr., Cuculis JJ et al. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 1970;18:315–333.
Chilosi M, Lestani M, Pedron S et al. A rapid immunostaining method for frozen sections. Biotech Histochem 1994;69:235–239.
Sabattini E, Bisgaard K, Ascani S et al. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol 1998;51:506–511.
Nallur G, Luo C, Fang L et al. Signal amplification by rolling circle amplification on DNA microarrays. Nucleic Acids Res 2001;29:E118.
Gusev Y, Sparkowski J, Raghunathan A et al. Rolling circle amplification: a new approach to increase sensitivity for immunohistochemistry and flow cytometry. Am J Pathol 2001;159:63–69.
Angel CE, George E, Brooks AE et al. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J Immunol 2006;176:5730–5734.
Brown J, Wimberly H, Lannin DR et al. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res 2014;20:5995–6005.
Farstad IN, Malavasi F, Haraldsen G et al. CD38 is a marker of human lacteals. Virchows Arch 2002;441:605–613.
Hudson DL, Guy AT, Fry P et al. Epithelial cell differentiation pathways in the human prostate: identification of intermediate phenotypes by keratin expression. J Histochem Cytochem 2001;49:271–278.
Gentry SM, Levenson RM . Biomedical applications of the information-efficient spectral imaging sensor (ISIS). Proc SPIE 3603, Systems and Technologies for Clinical Diagnostics and Drug Discovery II, 129 (21 April 1999); doi:10.1117/12.346734.
Levenson RM, Cronin PJ, Pankratov KK . Spectral imaging for brightfield microscopy. Proc SPIE 4959, Spectral Imaging: Instrumentation, Applications, and Analysis II, 27 (2 July 2003); doi:10.1117/12.485550.
Nadrigny F, Rivals I, Hirrlinger PG et al. Detecting fluorescent protein expression and co-localisation on single secretory vesicles with linear spectral unmixing. Eur Biophys J 2006;35:533–547.
Dickinson ME, Bearman G, Tille S et al. Multi-spectral imaging and linear unmixing add a whole new dimension to laser scanning fluorescence microscopy. Biotechniques 2001;31:1272 4-6, 8.
Mansfield JR, Hoyt C, Levenson RM . Visualization of microscopy-based spectral imaging data from multi-label tissue sections. Curr Protoc Mol Biol 2008, Chapter 14: Unit 14.9.
Gerdes MJ, Sevinsky CJ, Sood A et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci USA 2013;110:11982–11987.
Schubert W, Dress A, Ruonala M et al. Imaging cycler microscopy. Proc Natl Acad Sci USA 2014;111:E215.
Dabbs DJ . Diagnostic Immunohistochemistry. Elsevier Health Sciences, Philadelphia, PA, 2013.
Hammond ME, Hayes DF, Dowsett M et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–2795.
Wolff AC, Hammond ME, Hicks DG et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013.
Wolff AC, Hammond ME, Schwartz JN et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007;25:118–145.
Coons AH, Creech HJ, Jones RN . Immunological properties of an antibody containing a fluorescent group. Exp Biol Med 1941;47:200–202.
Marrack J . Nature of antibodies. Nature 1934;133:292–293.
Jensen EC . Types of imaging, Part 2: an overview of fluorescence microscopy. Anat Rec (Hoboken) 2012;295:1621–1627.
Spigel DR, Edelman MJ, O'Byrne K et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. J Clin Oncol 2014;32:5s.
Gustavson MD, Rimm DL, Dolled-Filhart M . Tissue microarrays: leaping the gap between research and clinical adoption. Personalized Med 2013;10:441–451.
Rojo MG, Bueno G, Slodkowska J . Review of imaging solutions for integrated quantitative immunohistochemistry in the Pathology daily practice. Folia Histochem Cytobiol 2009;47:349–354.
Schalper KA, Brown J, Carvajal-Hausdorf D et al. Objective measurement and clinical significance of tumor infiltrating lymphocytes in non-small cell lung cell cancer (NSCLC). J Natl Cancer Inst, in press.
Schubert W, Bonnekoh B, Pommer AJ et al. Analyzing proteome topology and function by automated multidimensional fluorescence microscopy. Nat Biotechnol 2006;24:1270–1278.
Clarke GM, Zubovits JT, Shaikh KA et al. A novel, automated technology for multiplex biomarker imaging and application to breast cancer. Histopathology 2014;64:242–255.
Angelo M, Bendall SC, Finck R et al. Multiplexed ion beam imaging of human breast tumors. Nat Med 2014;20:436–442.
Giesen C, Wang HA, Schapiro D et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods 2014;11:417–422.
Qian X, Lloyd RV . Recent developments in signal amplification methods for in situ hybridization. Diagn Mol Pathol 2003;12:1–13.
Yang H, Wanner IB, Roper SD et al. An optimized method for in situ hybridization with signal amplification that allows the detection of rare mRNAs. J Histochem Cytochem 1999;47:431–446.
Cassidy A, Jones J . Developments in in situ hybridisation. Methods 2014;70:39–45.
Jehan Z, Uddin S, Al-Kuraya KS . In-situ hybridization as a molecular tool in cancer diagnosis and treatment. Curr Med Chem 2012;19:3730–3738.
Kwon S . Single-molecule fluorescence in situ hybridization: quantitative imaging of single RNA molecules. BMB Rep 2013;46:65–72.
Liehr T, Weise A, Hamid AB et al. Multicolor FISH methods in current clinical diagnostics. Expert Rev Mol Diagn 2013;13:251–255.
Wang F, Flanagan J, Su N et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2012;14:22–29.
Raj A, Tyagi S . Detection of individual endogenous RNA transcripts in situ using multiple singly labeled probes. Methods Enzymol 2010;472:365–386.
Raj A, van den Bogaard P, Rifkin SA et al. Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods 2008;5:877–879.
Jakobsen MR, Bak RO, Andersen A et al. IFI16 senses DNA forms of the lentiviral replication cycle and controls HIV-1 replication. Proc Natl Acad Sci USA 2013;110:E4571–E4580.
Malone CD, Mestdagh C, Akhtar J et al. The exon junction complex controls transposable element activity by ensuring faithful splicing of the piwi transcript. Genes Dev 2014;28:1786–1799.
McIsaac RS, Silverman SJ, Parsons L et al. Visualization and analysis of mRNA molecules using fluorescence in situ hybridization in Saccharomyces cerevisiae. J Vis Exp, advance online publication, 14 January 2013; doi:10.3791/50382.
O'Grady T, Cao S, Strong MJ et al. Global bidirectional transcription of the Epstein-Barr virus genome during reactivation. J Virol 2014;88:1604–1616.
Pernicova Z, Slabakova E, Fedr R et al. The role of high cell density in the promotion of neuroendocrine transdifferentiation of prostate cancer cells. Mol Cancer 2014;13:113.
Rantala JK, Kwon S, Korkola J et al. Expanding the diversity of imaging-based RNAi screen applications using cell spot microarrays. Microarrays 2013;2:97–114.
Vera M, Pani B, Griffiths LA et al. The translation elongation factor eEF1A1 couples transcription to translation during heat shock response. eLife 2014;3:e03164.
Yang L, Lin C, Liu W et al. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell 2011;147:773–788.
Singel SM, Batten K, Cornelius C et al. Receptor-interacting protein kinase 2 promotes triple-negative breast cancer cell migration and invasion via activation of nuclear factor-kappaB and c-Jun N-terminal kinase pathways. Breast Cancer Res 2014;16:R28.
Singel SM, Cornelius C, Zaganjor E et al. KIF14 promotes AKT phosphorylation and contributes to chemoresistance in triple-negative breast cancer. Neoplasia 2014;16:247–256 56.e2.
Darnell DK, Stanislaw S, Kaur S et al. Whole mount in situ hybridization detection of mRNAs using short LNA containing DNA oligonucleotide probes. RNA 2010;16:632–637.
Robertson KL, Thach DC . LNA flow-FISH: a flow cytometry-fluorescence in situ hybridization method to detect messenger RNA using locked nucleic acid probes. Anal Biochem 2009;390:109–114.
Gupta A, Mo YY . Detection of microRNAs in cultured cells and paraffin-embedded tissue specimens by in situ hybridization. Methods Mol Biol 2011;676:73–83.
Nuovo GJ . In situ detection of microRNAs in paraffin embedded, formalin fixed tissues and the co-localization of their putative targets. Methods 2010;52:307–315.
Hanna JA, Wimberly H, Kumar S et al. Quantitative analysis of microRNAs in tissue microarrays by in situ hybridization. Biotechniques 2012;52:235–245.
Hanna JA, Hahn L, Agarwal S et al. in situ measurement of miR-205 in malignant melanoma tissue supports its role as a tumor suppressor microRNA. Lab Invest 2012;92:1390–1397.
Huang J, Zhou N, Watabe K et al. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1). Cell Death Dis 2014;5:e1008.
Liu Q, Huang J, Zhou N et al. LncRNA loc285194 is a p53-regulated tumor suppressor. Nucleic Acids Res 2013;41:4976–4987.
Liu X, Sempere LF, Ouyang H et al. MicroRNA-31 functions as an oncogenic microRNA in mouse and human lung cancer cells by repressing specific tumor suppressors. J Clin Invest 2010;120:1298–1309.
Nie Y, Liu X, Qu S et al. Long non-coding RNA HOTAIR is an independent prognostic marker for nasopharyngeal carcinoma progression and survival. Cancer Sci 2013;104:458–464.
Quesne JL, Jones J, Warren J et al. Biological and prognostic associations of miR-205 and let-7b in breast cancer revealed by in situ hybridization analysis of micro-RNA expression in arrays of archival tumour tissue. J Pathol 2012;227:306–314.
Sempere LF, Christensen M, Silahtaroglu A et al. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res 2007;67:11612–11620.
Sempere LF, Korc M . A method for conducting highly sensitive microRNA in situ hybridization and immunohistochemical analysis in pancreatic cancer. Methods Mol Biol 2013;980:43–59.
Yamamichi N, Shimomura R, Inada K et al. Locked nucleic acid in situ hybridization analysis of miR-21 expression during colorectal cancer development. Clin Cancer Res 2009;15:4009–4016.
Lizardi PM, Huang X, Zhu Z et al. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat Genet 1998;19:225–232.
Nilsson M, Krejci K, Koch J et al. Padlock probes reveal single-nucleotide differences, parent of origin and in situ distribution of centromeric sequences in human chromosomes 13 and 21. Nat Genet 1997;16:252–255.
Nilsson M, Malmgren H, Samiotaki M et al. Padlock probes: circularizing oligonucleotides for localized DNA detection. Science 1994;265:2085–2088.
Larsson C, Grundberg I, Soderberg O et al. In situ detection and genotyping of individual mRNA molecules. Nat Methods 2010;7:395–397.
Nilsson M, Barbany G, Antson DO et al. Enhanced detection and distinction of RNA by enzymatic probe ligation. Nat Biotechnol 2000;18:791–793.
Weibrecht I, Lundin E, Kiflemariam S et al. In situ detection of individual mRNA molecules and protein complexes or post-translational modifications using padlock probes combined with the in situ proximity ligation assay. Nat Protoc 2013;8:355–372.
Ke R, Mignardi M, Pacureanu A et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat Methods 2013;10:857–860.
Fitzgibbons PL, Bradley LA, Fatheree LA et al. Principles of analytic validation of immunohistochemical assays: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med 2014;138:1432–1443.
U. S. Food and Drugs Administration. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools) http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm2014, Accessed: 22 October 2014.
Anagnostou VK, Welsh AW, Giltnane JM et al. Analytic variability in immunohistochemistry biomarker studies. Cancer Epidemiol Biomarkers Prev 2010;19:982–991.
Bordeaux JM, Cheng H, Welsh AW et al. Quantitative in situ measurement of estrogen receptor mRNA predicts response to tamoxifen. PLoS One 2012;7:e36559.
Giltnane JM, Rimm DL . Technology insight: identification of biomarkers with tissue microarray technology. Nat Clin Pract Oncol 2004;1:104–111.
McCabe A, Dolled-Filhart M, Camp RL et al. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst 2005;97:1808–1815.
Welsh AW, Moeder CB, Kumar S et al. Standardization of estrogen receptor measurement in breast cancer suggests false-negative results are a function of threshold intensity rather than percentage of positive cells. J Clin Oncol 2011;29:2978–2984.
Cheang MC, Treaba DO, Speers CH et al. Immunohistochemical detection using the new rabbit monoclonal antibody SP1 of estrogen receptor in breast cancer is superior to mouse monoclonal antibody 1D5 in predicting survival. J Clin Oncol 2006;24:5637–5644.
Welsh AW, Harigopal M, Wimberly H et al. Quantitative analysis of estrogen receptor expression shows SP1 antibody is more sensitive than 1D5. Appl Immunohistochem Mol Morphol 2013;21:139–147.
Brock JE, Hornick JL, Richardson AL et al. A comparison of estrogen receptor SP1 and 1D5 monoclonal antibodies in routine clinical use reveals similar staining results. Am J Clin Pathol 2009;132:396–401.
Ratner M . FDA pushes for control over laboratory-developed tests. Nat Biotechnol 2014;32:855.
Hayes DF, Bast RC, Desch CE et al. Tumor marker utility grading system: a framework to evaluate clinical utility of tumor markers. J Natl Cancer Inst 1996;88:1456–1466.
Simon RM, Paik S, Hayes DF . Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009;101:1446–1452.
Gettinger S, Herbst RS . B7-H1/PD-1 blockade therapy in non-small cell lung cancer: current status and future direction. Cancer J 2014;20:281–289.
Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443–2454.
Schalper KA . PD-L1 expression and tumor-infiltrating lymphocytes: revisiting the antitumor immune response potential in breast cancer. Oncoimmunology 2014;3:e29288.
Schalper KA, Velcheti V, Carvajal D et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 2014;20:2773–2782.
Khambata-Ford S, Harbison CT, Hart LL et al. Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2010;28:918–927.
Brown JR, DiGiovanna MP, Killelea B et al. Quantitative assessment Ki-67 score for prediction of response to neoadjuvant chemotherapy in breast cancer. Lab Invest 2014;94:98–106.
Catenacci DV, Liao WL, Thyparambil S et al. Absolute quantitation of Met using mass spectrometry for clinical application: assay precision, stability, and correlation with MET gene amplification in FFPE tumor tissue. PLoS One 2014;9:e100586.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
DL Rimm is a consultant to a number of companies. Gilead Sciences and Kolltan support work in the lab of DL Rimm but this study was not supported by these sources.
Additional information
This review describes methods for visualization and quantification of biomarkers (protein and mRNA) in tissue. The authors then consider the challenges in bringing quantitative tissue biomarkers to the clinic.
Rights and permissions
About this article
Cite this article
Carvajal-Hausdorf, D., Schalper, K., Neumeister, V. et al. Quantitative measurement of cancer tissue biomarkers in the lab and in the clinic. Lab Invest 95, 385–396 (2015). https://doi.org/10.1038/labinvest.2014.157
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2014.157
This article is cited by
-
Selection of optimal quantile protein biomarkers based on cell-level immunohistochemistry data
BMC Bioinformatics (2023)
-
Bile acid distributions, sex-specificity, and prognosis in colorectal cancer
Biology of Sex Differences (2022)
-
QuantISH: RNA in situ hybridization image analysis framework for quantifying cell type-specific target RNA expression and variability
Laboratory Investigation (2022)
-
Rapid micro-immunohistochemistry
Microsystems & Nanoengineering (2020)
-
Analysis of Immune Checkpoint Drug Targets and Tumor Proteotypes in Non-Small Cell Lung Cancer
Scientific Reports (2020)