Abstract
Glioblastoma (GBM) patients have dismal median survival even with the most rigorous treatments currently available. Radiotherapy is the most effective non-surgical therapy for GBM patients; however, patients succumb due to tumor recurrence within a year. To develop a curative therapeutic approach, we need to better understand the underlying molecular mechanism of radiation resistance in GBM. Towards this goal, we developed an in vivo orthotopic GBM model system that mimics the radiation response of human GBM, using both established-GBM cell line and patient-derived freshly dissociated GBM specimen. In-vivo ionizing radiation (IR) treatment prolonged the survival of mice with intracranical tumor derived from U373MG, but failed to prevent tumor recurrence. U373MG and GBM578 cells isolated after in-vivo IR (U373-IR and 578-IR) were more clonogenic and enriched with stem cell-like characteristics, compared with mock-treated control tumor cells. Transcriptomic analyses and quantitative real-time reverse-transcription PCR analyses using these matched GBM cells before and after radiation treatment revealed that Wnt pathways were preferentially activated in post-IR GBM cells. U373-IR cells and 578-IR were enriched with cells positive for both active β-catenin (ABC) and Sox2 population, and this subpopulation was further increased after additional in-vitro radiation treatment, suggesting that radiation resistance of GBM is mediated due, in part, to the activation of stem cell-associated pathways including Wnt. Finally, pharmacological and siRNA inhibition of Wnt pathway significantly decreased the survival and clonogenicity of GBM cells and reduced their ABC+/Sox2+ population. Together, these data suggest that Wnt activation is a molecular mechanism to confer GBM radioresistance and an important therapeutic target.
Similar content being viewed by others
Main
Glioblastoma (GBM) is the most common and lethal brain cancer. The most effective therapy available to GBM patients now consists of maximal surgical resection followed by concurrent radiation, and the oral methylator temozolomide. Unfortunately, these aggressive treatments and more recent molecularly targeted therapeutics provide only palliation, unable to change the near uniform lethality of this disease. For the development of curative therapy, it is crucial to identify the mechanisms involved in treatment resistance.
Recent evidence suggests that GBMs contain a subpopulation that is highly enriched with stem cell-like characteristics and tumor propagating capacity. Although some cancers may not follow cancer stem cell model, numerous studies including ours suggests that GBM stem cells (GSCs) are responsible for glioma propagation, resistance to conventional therapy, and tumor recurrence. CD133 (also known as PROM1) was first proposed as an enrichment marker for GSCs,1, 2 but the universality of this marker is limited, as later studies have reported that some GBM do not follow CD133 hierarchy.3, 4, 5 Subsequent studies have identified additional GSC enrichment markers including L1CAM,6 CD15,7 A2B5,8 integrin alpha 6,9 and CD44.10 Yet, most of these GSC enrichment markers, although useful for the prospective isolation, do not have distinct functional roles pertinent to GSC biology. Therefore, regardless of the status of the GSC surface marker(s), it would be important to gain a functional insight into which a certain cellular mechanism(s) endow treatment resistance to GBM.
In this study, we generated orthotopic GBM xenograft models to study the mechanism of radiation resistance. We chose in vivo radiation models to better represent brain microenvironment,11 thereby making it more clinically relevant. Multimodality studies using both in vivo and in vitro radiation revealed a key role of Wnt activation in GBM radiation resistance.
MATERIALS AND METHODS
Isolation of Primary GBM Cells
Following informed consent and in accordance with the appropriate Institutional Review Boards, GBM specimen 578 (GBM578) was obtained from a patient undergoing surgery at the Samsung Medical Center. The tumor sample was classified as GBM on the basis of World Health Organization criteria by examination of pathologists.12 Within an hour of surgical resection, the tumor was mechanically and enzymatically dissociated into single cells, according to previous reports.13, 14 GBM578 was briefly maintained in NBE neurosphere culture medium14 before injection in mice: Neurobasal-A medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2 mM glutamine (Invitrogen), 100 units per ml penicillin, 100 μg/ml streptomycin (Invitrogen), recombinant human basic fibroblast and epidermal growth factors (50 ng/ml each; R&D Systems), N2 and B27 supplements (0.5 × each; Invitrogen).
Cell Culture
U87MG and U373MG were purchased from the American Tissue Culture Collection. For xenograft tumor formation, these cells were expanded in the standard recommended medium, Dulbecco's modified Eagle's medium supplemented with 10% v/v fetal bovine serum, 100 units per ml penicillin, and 100 μg/ml streptomycin. All ex-vivo cells dissociated from mouse brains (U373-C, U373-IR, U87-C, U87-IR, 578-C, and 578-IR) were maintained in NBE.
Orthotopic GBM Xenograft Models
Six-week-old male BALB/c nude mice (Orient Bio, Seoul, South Korea) were used for intracranial transplanation. Animals were given either U87MG (2 × 105 per mouse) or U373MG (1 × 105 per mouse) glioma cells by stereotactic intracranial injection (coordinates: 2 mm anterior, 2 mm lateral, 2.5 mm depth from the dura). These cells generated tumors in a highly reproductive manner. At 75% of median survival (U87MG, 21 days; U373MG, 16 days), mice received either mock or 10 Gy whole-brain ionizing radiation (IR). Mice were killed either when 25% body weight loss or neurological symptoms (lethargy, ataxia, and seizures) were observed. For survival analyses, seven mice were used per group. For obtaining cells from the xenograft tumors, five mice per group were sacrificed 5 days post in-vivo radiation treatment. All mice experiments were performed according to the Association for Assessment and Accreditation of Laboratory Animal Care-accredited guidelines of our institute's Animal Use and Care Committee.
Clonogenic Assay
Soft agar clonogenic assay was performed in six-well plates with Noble agar as previously described,15 with 0.8%. (w/v) bottom and 0.4% (w/v) top layers. For Wnt signaling inhibition, 5 μM XAV939 (Tocris Bioscience, Ellisville, MO, USA) was added in the agar media. Cells were irradiated with doses ranging up to 10 Gy and then seeded at the density of 1000 cells per well in the top layer. After 2 weeks, colonies were stained with 0.01% crystal violet, and those with at least 50 cells were counted manually under a microscope.
Limiting Dilution Assay
Limiting dilution assay (LDA) was performed in 96-well plates as previously described.16 Cells were irradiated at 0 or 4 Gy and seeded at a range of 5–500 cells per well. After 1 week, the number of wells without spheres was counted. LDA clonogenic index was calculated as the inverse of the x-intercept of the regression between the number of wells without spheres and the number of cells seeded.
Microarray Analysis
Gene expression microarray was performed using SurePrint G3 Human Expression 8 × 60 k Kit (Agilent Technologies, Santa Clara, CA, USA). RNAs were extracted using Trizol (Invitrogen) and processed to acquire fluorescent complementary RNAs (Agilent's Low Input Quick Amp Labeling Kit). These labeled RNAs were purified (Qiagen, Valencia, CA, USA) and hybridized to Agilent Whole Human Genome Oligo Microarrays according to manufacturer's instructions (Agilent Technologies). Data were processed and analyzed by GeneSpring GX version 11.5 (Agilent Technologies).
qRT–PCR Analysis
RNAs were extracted (Qiagen) and their complementary DNAs were synthesized (Invitrogen) per manufacturers’ instructions. Quantitative reverse-transcriptase PCR (qRT–PCR) was performed using primers listed in Supplementary Table S1 in LightCycler 480 (Roche Diagnostics, Indianapolis, IN, USA). Duplicate reactions were performed for each set of primers.
Flow Cytometry Analysis
The following antibodies were conjugated to PerCP-Cy5.5. as per the manufacturer's instructions (AbD Serotec, Oxford, UK): anti-active β-catenin (ABC; Millipore, Billerica, MA, USA), anti-Sox2 (R&D Systems), and isotype control anti-mouse IgG (BD Biosciences, San Jose, CA, USA). Cells were fixed and then permeabilized for intracellular staining using solutions from BD Biosciences. For dual staining, ABC and Sox2 were detected indirectly with Alexa Fluor 488 (Invitrogen) and directly with conjugated PerCP-Cy5.5, respectively. For Wnt signaling inhibition, 5 μM XAV939 was added to culture media 16 h before analysis. Flow cytometry was performed in FACS Calibur (BD Biosciences). Anti-MHC class I H-2Kd antibody specific for mouse (BD Biosciences) was used to determine the purity of human-origin tumor cells in ex-vivo cultures.
Gene Transfection
Lipofectamine 2000 was used for gene transfection, according to the manufacturer's instructions (Invitrogen). Non-targeting and β-catenin siRNAs were designed (Supplementary Table S2; Genolution Pharmaceuticals, Seoul, South Korea), and 600 pmol of each were transfected in 100-mm dishes. Mock plasmid DNA (pcDNA3.1) of 10 μg or constitutively active β-catenin (S45Y mutation) plasmid DNA (generous gift from Sung Hee Baek, Seoul National University) was transfected in 100-mm dishes.
RESULTS
Model Generation and Radiation-Resistant Colony Selection
To better understand the molecular mechanism of radiation resistance in GBM, we developed an in vivo orthotopic glioma model system that mimics the radiation response in the clinic. The mice bearing xenograft tumors derived from U87MG, U373MG, and primary GBM578 had median survival of 28, 21, and 56 days, respectively. Single 10 Gy in vivo whole-brain IR was given to the three models at about 75% of median survival (21, 16, and 49 days, respectively). In-vivo IR treatment significantly prolonged the survival of mice bearing U373 xenograft (23%, P<0.02 by log-rank test), whereas the treatment had no effect on mice with U87MG xenografts (P>0.05; Figure 1a, b).
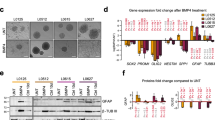
Xenograft model of U87MG and U373MG. (a) Schedule of radiation therapy in intracranial xenograft models generated with U87MG (2 × 105 per mouse), U373MG (1 × 105 per mouse), and GBM578 (5 × 104 per mouse; median survival of 28, 21, and 56 days, respectively). (b) Survival analysis of U87MG (left panel) and U373MG (right panel) xenografts with either mock (c) or 10 Gy whole-brain ionizing radiation (IR). (c) Soft agar clonogenic assay of parental cell lines (left panel; square=U87MG, circle=U373MG), U87MG ex-vivo cell lines (middle panel; open square=U87-C, closed square=U87-IR), and U373MG ex-vivo cell lines (right panel; open circle=U373-C, closed circle=U373-IR). Abbreviations: IC=intracranial, C=control (mock IR). (d) Limiting dilution assay (LDA) of ex-vivo cells from GBM578 xenografts treated with mock (578-C) or 10 Gy IR (578-IR). LDA clonogenic index is measured as the inverse of the x-intercept; higher number indicates higher colony-forming efficiency. 578-IR have higher baseline colony-forming efficiency than 578-C (P<0.05).
This differential effect of in-vivo IR can be explained by the difference in intrinsic radioresistance between the two glioma cells (Figure 1). To test, we preformed soft agar clonogenic assays using the parental cells, as well as the freshly dissociated ex-vivo cells from each xenograft tumor (Figure 1c). The ex-vivo cells were confirmed to be of human origin, and were not contaminated with mouse cells (Supplementary Figure S1). Clonogenicity of the parental U87MG cells was similar to that of U87-C or U87-IR (Figure 1c; compare the square symbols in the left and middle panels). In sharp contrast, U373-IR cells generated much more colonies than the parental and U373-C cells (Figure 1c; compare circles in the leftmost and rightmost panels of Figure 1c). For the cells derived from primary GBM578, we employed LDA (also known as sphere formation assay) as a surrogate clonogenic assay, arguably a more appropriate assay for cells grown in GSC condition employed here.16 In-vivo irradiated GBM578 (578-IR) was observed to have higher sphere colony-forming efficiency than mock-treated GBM578 (578-C), per LDA clonogenic index measurement (Figure 1d; P<0.05). These data suggest that in vivo irradiation either changes the cellular phenotype towards increased radioresistance or induces the enrichment of more radioresistant cells.
Microarray Analysis Reveals Upregulated Wnt Signaling in U373-IR Tumor
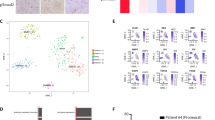
To interrogate the underlying mechanism(s) of radioresistance, we first performed transcriptomic analysis using xenograft tumors with or without in vivo-IR (n=7 each). U373-C and U373-IR revealed significant changes in gene expression. Over 2400 genes were found to have significantly altered expression (1082 upregulated and 1360 downregulated, P<0.05 ANOVA; partial list in Table 1). These genes belong to multiple Gene Ontology categories, including those implicated in development, cell differentiation, receptor activity, and signaling pathways (Figure 2).
In particular, expression changes in Wnt signaling genes were highly significant. For example, WISP1, an effector of activated Wnt signaling previously reported to regulate cell survival,17, 18, 19 showed the highest level change (3.6-fold higher in U373-IR than in U373-C). Patient data at the US National Cancer Institute (Repository of Molecular Brain Neoplasia Data (REMBRANDT)20, 21) also indicated that upregulation of WISP1 is associated with poorer clinical outcome (Supplementary Figure S2), further substantiating our data. qRT–PCR of Wnt signaling genes corroborated with the microarray data (Figure 3). In sum, these data suggest that Wnt signaling is more elevated in U373-IR.
U373-IR and 578-IR have Higher Levels of Active β-catenin and Stem Cell Markers
To further confirm the Wnt activation status in U373-IR and 578-IR, we determined the expression of ABC (dephosphorylated Ser37 and Thr41), a representative marker for activated Wnt signaling,22 using flow cytometry. Consistent with the mRNA data (Figure 3), U373-IR and 578-IR cells had higher ABC mean fluorescence intensity (MFI) than the U373-C and 578-C cells, respectively (Figure 4; P<0.01). Interestingly, U87-C and U87-IR cells had significantly higher baseline expression of ABC, providing corroborating evidence for their higher radioresistant character seen in Figure 1.
Protein level confirmation of activated Wnt signaling and stem cell markers using flow cytometry. (a) Fixed and permeabilized U373-C, U373-IR, U87-C, U87-IR, 578-C, and 578-IR cells were probed with active β-catenin (ABC) and Sox2 antibodies. There was a significant difference in the percent positive and in the mean fluorescence intensity (MFI; P<0.05 and 0.01 Student's t-test, respectively). Dotted line=control, solid line=ionizing radiation (IR). (b) Dual staining of active β-catenin and Sox2 of U373-C, U373-IR, U87-C, U87-IR, 578-C, and 578-IR. Quadrants were drawn based on isotype negative-control staining (not shown).
Given that Wnt signaling is implicated in stem cell-like phenotype and that radioresistant GSCs have higher expression of stem cell-associated proteins, we hypothesized that U373-IR and 578-IR cells have elevated expression of stem cell proteins. To test, we performed flow cytometry using antibodies against ABC and Sox2. IR groups showed higher MFI for both markers (Figure 4a; P<0.01). Next, we performed dual flow cytometry analysis to determine whether a subpopulation positive for both antibodies exists, and whether this population is enriched in the IR cells. The percentage of ABC+/Sox2+ cells was much higher in U373-IR cells than non-radiated control cells (U373-C 40.5% vs U373-IR 87.6%; P<0.05). Similarly, patient-derived 578-IR cells had higher ABC+/Sox2+ cells (578-C 1.5% vs 578-IR 18.6%; P<0.05). Taken together, these data suggest the elevated expression of Wnt activation, and stem cell proteins in U373-IR and 578-IR might confer the increased radioresistant phenotyope of these cells.
Inhibition of Wnt Sensitized U373-IR to Radiation
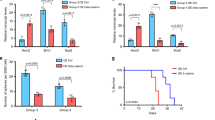
As a final verification that Wnt signaling endows U373-IR cells an increased radiation resistance, clonogenic assay was performed in the presence of XAV939, a Wnt signaling pathway inhibitor.23 The addition of XAV939 decreased the Sox2+/ABC+ population from 40% to 24% and 67% to 43%, respectively, for U373-C and U373-IR (Figure 5a; P<0.05). In addition, the combination of XAV939 and in vitro IR significantly decreased the surviving fraction of glioma cells, supporting the notion that Wnt targeting as a potential radiosensitizer (Figure 5b, compare with Figure 1c). To exclude the possibility of off-target effects by XAV939, we performed siRNA-mediated knockdown experiments. Similar to XAV939-treated cells, U373 ex-vivo cells transfected with β-catenin siRNA were much more sensitive to radiation than the cells with control siRNA (Figure 5c). Conversely, overexpression of constitutively active β-catenin mutant in these cells increased their radioresistance (Supplementary Figure S3). Together, these data suggest that the radiosensitization conferred by Wnt signaling inhibition occurs concurrently with the decrease of stem cell-like population.
Radiosensitization by inhibiting Wnt signaling. (a) Sox2 and active β-catenin (ABC) dual staining of U373-C and U373-IR, with or without 16 h treatment with Wnt pathway inhibitor XAV939 (5 μM). There was a significant difference between the groups (P<0.05 Student's t-test). (b) Corresponding soft agar clonogenic assay of U373-C (squares) and U373-IR (circles) with (closed symbols) or without (open symbols) the addition of XAV939 (5 μM). (c) Soft agar clonogenic assay of U373-C (squares) and U373-IR (circles) transfected with non-targeting siRNA (open symbols) or with β-catenin siRNA (closed symbols).
DISCUSSION
Developmental pathways, including the Wnt pathway, have long been postulated to have a role in the maintenance of self-renewing cancer stem cells.22, 24, 25 The role of Wnt pathway has been shown in colon and breast cancers,26, 27 and a recent study in breast cancer have reported that radiation enriches stem cell-like breast progenitor cells with highly activated Wnt signaling.28 Wnt pathways in GBM have been investigated by a few groups including ours; however, the role of Wnt in GBM has remained largely hypothetical. Here, we presented evidence to support the role of Wnt in GBM propagation and radioresistance, by employing in vitro and in vivo radiation approaches. Our results corroborate with a recent report showing the high expression of β-catenin is correlated with poor prognosis in GBM patients.29
Our previous study with a highly invasive GBM cell line provided initial evidence of Wnt's role in stem cell-like characteristics.30 Here, we further showed that these stem cell-like GBM cells possess high-level expressions of active β-catenin and Sox2 that were enriched in the radiation-resistant cells (Figure 4). It raises an interesting possibility that the ABC+/Sox2+ population function as GSC-like cells, and that the invasiveness and radioresistance are shared phenotypes of Wnt signaling. Importance of targeting invasiveness to increase the effectiveness of radiotherapy was suggested before,31, 32 but the exact contribution of Wnt to radiation-induced invasiveness remains to be elucidated.
Our expression arrays of U373-C and U373-IR revealed the significant enrichment of several other pathways that are implicated in oncogenic process, developmental pathways, and DNA damage responses. In response to radiation, others have reported that GSCs have robust DNA damage response2, 6, 33, 34 compared with the bulk tumor, and have suggested that targeting the DNA checkpoint kinases, such as Chk1/2, as a potential therapeutic approach against GBM. Other studies also reported the importance of Notch and c-Met signaling in GBM radioresistance.16, 35 Our preliminary proteomics analysis also implicated the activation of autophagy and epithelial–mesenchymal transition in the radioresistant cells (data not shown). Although the former corroborates with a previous study that showed that autophagy contributes to radioresistance of GSCs,36 the latter, although generally hypothesized in cancer stem cells, has not been demonstrated yet in glioma.32, 37, 38 Taken together, the implication is that Wnt is one of many important pathways involved in GBM radioresistance, and future studies that link these pathways (Wnt, Notch, c-Met, DNA checkpoint, autophagy, and epithelial–mesenchymal transition) are warranted. Our results presented here implicate Wnt inhibition as one possible radiosensitizing agent (Figure 5), and we speculate that a combination therapy that inhibit some or all of the other mentioned pathways will likely translate to successful treatment in the clinic.
Our in vivo radiation model system described here has several limitations. First, although we provided results derived from freshly isolated tumor cells from a single human GBM specimen (GBM578) that retain the biology of primary GBM in situ, the use of additional specimens will further substantiate the findings of this study. In addition, we only applied single-dose IR, rather than the clinical practice of multiple low dosing. Notwithstanding these caveats, our model systems serve as a highly reproducible model for studying the effects of radiation in vivo. We identified a few Wnt signaling-related genes that were previously unrecognized in gliomas and confirmed that expression of each gene, for example WISP1, portends the poor survival of GBM patients (Supplementary Figure S2). In sum, our data indicate a critical role of Wnt signaling in GBM radiation resistance and provide preclinical evidence to suggest a Wnt inhibition as a potential radiosensitizer.
References
Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature 2004;432:396–401.
Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006;444:756–760.
Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res 2007;67:4010–4015.
Wang J, Sakariassen PO, Tsinkalovsky O, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer 2008;122:761–768.
Joo KM, Kim SY, Jin X, et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest 2008;88:808–815.
Cheng L, Wu Q, Huang Z, et al. L1CAM regulates DNA damage checkpoint response of glioblastoma stem cells through NBS1. EMBO J 2011;30:800–813.
Son MJ, Woolard K, Nam DH, et al. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell 2009;4:440–452.
Tchoghandjian A, Baeza N, Colin C, et al. A2B5 cells from human glioblastoma have cancer stem cell properties. Brain Pathol 2010;20:211–221.
Lathia JD, Gallagher J, Heddleston JM, et al. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 2010;6:421–432.
He J, Liu Y, Xie X, et al. Identification of cell surface glycoprotein markers for glioblastoma-derived stem-like cells using a lectin microarray and LC-MS/MS approach. J Proteome Res 2010;9:2565–2572.
Jamal M, Rath BH, Williams ES, et al. Microenvironmental regulation of glioblastoma radioresponse. Clin Cancer Res 2010;16:6049–6059.
Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97–109.
Joo KM, Nam DH . Prospective identification of cancer stem cells with the surface antigen CD133. Methods Mol Biol 2009;568:57–71.
Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006;9:391–403.
Franken NA, Rodermond HM, Stap J, et al. Clonogenic assay of cells in vitro. Nat Protoc 2006;1:2315–2319.
Wang J, Wakeman TP, Lathia JD, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells 2010;28:17–28.
Su F, Overholtzer M, Besser D, et al. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev 2002;16:46–57.
Venkatesan B, Prabhu SD, Venkatachalam K, et al. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal 2010;22:809–820.
Chen PP, Li WJ, Wang Y, et al. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One 2007;2:e534.
Madhavan S, Zenklusen JC, Kotliarov Y, et al. Rembrandt: helping personalized medicine become a reality through integrative translational research. Mol Cancer Res 2009;7:157–167.
National Cancer Institute. REMBRANDT home page. 2005. (cited 24 May 2011); Available from: http://rembrandt.nci.nih.gov.
Reya T, Clevers H . Wnt signalling in stem cells and cancer. Nature 2005;434:843–850.
Huang SM, Mishina YM, Liu S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009;461:614–620.
Beachy PA, Karhadkar SS, Berman DM . Tissue repair and stem cell renewal in carcinogenesis. Nature 2004;432:324–331.
Kim Y, Joo KM, Jin J, et al. Cancer stem cells and their mechanism of chemo-radiation resistance. Int J Stem Cells 2009;2:109–114.
Herbst A, Kolligs FT . Wnt signaling as a therapeutic target for cancer. Methods Mol Biol 2007;361:63–91.
de Sousa EM, Vermeulen L, Richel D, et al. Targeting Wnt signaling in colon cancer stem cells. Clin Cancer Res 2011;17:647–653.
Woodward WA, Chen MS, Behbod F, et al. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA 2007;104:618–623.
Rossi M, Magnoni L, Miracco C, et al. β-catenin and Gli1 are prognostic markers in glioblastoma. Cancer Biol Ther 2011;11:753–761.
Jin X, Jeon HY, Joo KM, et al. Frizzled 4 regulates stemness and invasiveness of migrating glioma cells established by serial intracranial transplantation. Cancer Res 2011;71:3066–3075.
Wild-Bode C, Weller M, Rimner A, et al. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res 2001;61:2744–2750.
Harless WW . Cancer treatments transform residual cancer cell phenotype. Cancer Cell Int 2011;11:1.
Chu PM, Chiou SH, Su TL, et al. Enhancement of radiosensitivity in human glioblastoma cells by the DNA N-mustard alkylating agent BO-1051 through augmented and sustained DNA damage response. Radiat Oncol 2011;6:7.
Silvestre DC, Pineda JR, Hoffschir F, et al. Alternative lengthening of telomeres in human glioma stem cells. Stem Cells 2011;29:440–451.
Lal B, Xia S, Abounader R, et al. Targeting the c-Met pathway potentiates glioblastoma responses to gamma-radiation. Clin Cancer Res 2005;11:4479–4486.
Lomonaco SL, Finniss S, Xiang C, et al. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer 2009;125:717–722.
Short JJ, Curiel DT . Oncolytic adenoviruses targeted to cancer stem cells. Mol Cancer Ther 2009;8:2096–2102.
Theys J, Jutten B, Habets R, et al. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol 2011;99:392–397.
Acknowledgements
We thank Mi-Young Jo, Wonyoung Kang, Younggeon Jin, Yeri Lee, Hye Mi Kim, Jisook Song, Youngju Yoon, Ki Haeng Lee, Kyoung Min Lee, and Hye Rin Lee for their technical support with the animal experiments and Bong Gu Kang for his critical input. We are also grateful to Sung Hee Baek (Seoul National University) for kindly providing the S45Y β-catenin plasmid DNA. This work was supported by the National Research Foundation of KOREA (NRF) grant funded by the Korea government (MEST; Grant number 20100029550) SDG.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
The Wnt signaling pathway is up-regulated in radiation-enriched glioblas toma stem cell-like cells, which makes them radioresistant; inhibition of Wnt signaling sensitizes the cells to radiation. Thus, targeting Wnt may assist radiation therapy in glioblastoma patients.
Rights and permissions
About this article
Cite this article
Kim, Y., Kim, K., Lee, J. et al. Wnt activation is implicated in glioblastoma radioresistance. Lab Invest 92, 466–473 (2012). https://doi.org/10.1038/labinvest.2011.161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2011.161
Keywords
This article is cited by
-
DHODH inhibition impedes glioma stem cell proliferation, induces DNA damage, and prolongs survival in orthotopic glioblastoma xenografts
Oncogene (2022)
-
WNT signaling modulates chemoresistance to temozolomide in p53-mutant glioblastoma multiforme
Apoptosis (2022)
-
Assessing fatty acid-induced lipotoxicity and its therapeutic potential in glioblastoma using stimulated Raman microscopy
Scientific Reports (2021)
-
Wnt signaling induces radioresistance through upregulating HMGB1 in esophageal squamous cell carcinoma
Cell Death & Disease (2018)
-
Synergistic effect of TRAIL and irradiation in elimination of glioblastoma stem-like cells
Clinical and Experimental Medicine (2018)