Abstract
Objective:
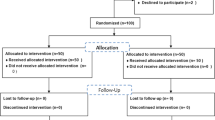
To compare two enoxaparin dosing strategies at achieving prophylactic anti-Xa levels in women with a body mass index (BMI) ⩾35 (kg m−2) postcesarean delivery.
Study Design:
Women with BMI ⩾35 were randomized to receive prophylactic enoxaparin at a fixed dose of 40 mg daily or weight-based dosing of 0.5 mg kg−1 twice daily. The primary outcome was the proportion of subjects with peak anti-Xa levels in the prophylactic range of 0.2 to 0.6 IU ml−1.
Result:
From August 2013 through February 2014, 84 demographically similar women completed the protocol. In the weight-based group, 88% (37/42) of the women reached prophylactic anti-Xa levels versus 14% (6/42) in the fixed dose group (odds ratio 44.4, 95% confidence interval 12.44, 158.48, P<0.001). No anti-Xa level exceeded 0.48 IU ml−1. There were no venous thromboembolic or bleeding events requiring reoperation or transfusion in either group.
Conclusion:
Compared with fixed dosing daily, weight-based dosing twice daily more effectively achieved prophylactic anti-Xa levels without reaching the therapeutic range.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Clark SL, Belfort MA, Dildy GA, Herbst MA, Meyers JA, Hankins GD . Maternal death in the 21st century: causes, prevention, and relationship to cesarean delivery. Am J Obstet Gynecol 2008; 199 (1): 36.e1–36.e5; discussion 91–2. e7–11.
Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ 3rd . Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Int Med 2005; 143 (10): 697–706.
Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, Elkind MS . Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med 2014; 370 (14): 1307–1315.
Sultan AA, West J, Tata LJ, Fleming KM, Nelson-Piercy C, Grainge MJ . Risk of first venous thromboembolism in and around pregnancy: a population-based cohort study. Br J Haematol 2012; 156 (3): 366–373.
Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD . Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. BJOG 2001; 108 (1): 56–60.
O American College of and Gynecologists. ACOG Committee opinion no. 549: obesity in pregnancy. Obstet Gynecol 2013; 121 (1): 213–217.
Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (2 Suppl): e691S–e736S.
Duhl AJ, Paidas MJ, Ural SH, Branch W, Casele H, Cox-Gill J et al. Antithrombotic therapy and pregnancy: consensus report and recommendations for prevention and treatment of venous thromboembolism and adverse pregnancy outcomes. Am J Obstet Gynecol 2007; 197 (5): 457.e1–457.e21.
A James and B-O Committee on Practice. Practice bulletin no. 123: thromboembolism in pregnancy. Obstet Gynecol 2011; 118 (3): 718–729.
Rondina MT, Wheeler M, Rodgers GM, Draper L, Pendleton RC . Weight-based dosing of enoxaparin for VTE prophylaxis in morbidly obese, medically-Ill patients. Thromb Res 2010; 125 (3): 220–223.
James AH, Jamison MG, Brancazio LR, Myers ER . Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006; 194 (5): 1311–1315.
RCoOa Gynaecologists. Green-top Guideline 37a: reducing the risk of thrombosis and embolism during pregnancy and the puerperium. 2015. Available from: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf.
ACoO Committee on Practice Bulletins—Gynecology and Gynecologists. ACOG Practice Bulletin No. 84: prevention of deep vein thrombosis and pulmonary embolism. Obstet Gynecol 2007; 110 (2 Pt 1): 429–440.
Ellison J, Thomson AJ, Conkie JA, McCall F, Walker D, Greer A . Thromboprophylaxis following caesarean section—a comparison of the antithrombotic properties of three low molecular weight heparins—dalteparin, enoxaparin and tinzaparin. Thromb Haemost 2001; 86 (6): 1374–1378.
Casele HL, Laifer SA, Woelkers DA, Venkataramanan R . Changes in the pharmacokinetics of the low-molecular-weight heparin enoxaparin sodium during pregnancy. Am J Obstet Gynecol 1999; 181 (5 Pt 1): 1113–1117.
Freeman A, Horner T, Pendleton RC, Rondina MT . Prospective comparison of three enoxaparin dosing regimens to achieve target anti-factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol 2012; 87 (7): 740–743.
Fox NS, Laughon SK, Bender SD, Saltzman DH, Rebarber A . Anti-factor Xa plasma levels in pregnant women receiving low molecular weight heparin thromboprophylaxis. Obstet Gynecol 2008; 112 (4): 884–889.
Malinoski D, Jafari F, Ewing T, Ardary C, Conniff H, Baje M et al. Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically ill trauma and surgical patients. J Trauma 2010; 68 (4): 874–880.
Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141 (2 Suppl): e227S–e277S.
Mishanina E, Rogozinska E, Thatthi T, Uddin-Khan R, Khan KS, Meads C . Use of labour induction and risk of cesarean delivery: a systematic review and meta-analysis. CMAJ 2014; 186 (9): 665–673.
Hiscock RJ, Casey E, Simmons SW, Walker SP, Newell PA . Peak plasma anti-Xa levels after first and third doses of enoxaparin in women receiving weight-based thromboprophylaxis following caesarean section: a prospective cohort study. Int J Obstet Anesth 2013; 22 (4): 280–288.
Borkgren-Okonek MJ, Hart RW, Pantano JE, Rantis PC Jr, Guske PJ, Kane JM Jr et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis 2008; 4 (5): 625–631.
Overcash RT, Lacoursiere DY . The clinical approach to obesity in pregnancy. Clin Obstet Gynecol 2014; 57 (3): 485–500.
FDA Drug Safety Communications: updated recommendations to decrease risk of spinal column bleeding and paralysis in patients on low molecular weight heparins. 2013. [cited 11-6-13] Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM373735.pdf.
Marrs CC, Moussa HN, Sibai BM, Blackwell SC . The relationship between primary cesarean delivery skin incision type and wound complications in women with morbid obesity. Am J Obstet Gynecol 2014; 210 (4): 319.e1–319.e4.
Alanis MC, Villers MS, Law TL, Steadman EM, Robinson CJ . Complications of cesarean delivery in the massively obese parturient. Am J Obstet Gynecol 2010; 203 (3): 271.e1–271.e7.
Acknowledgements
This study was conducted at the Memorial Care Center for Women at Miller Children’s Hospital, Long Beach Memorial Medical Center, Long Beach, CA 90806, USA and funded by a grant from the Long Beach Memorial Medical Center Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented at the 35th Annual Meeting of the Society for Maternal Fetal Medicine, San Diego, CA, USA, 2–7 February 2015, Abstract number 26.
Rights and permissions
About this article
Cite this article
Stephenson, M., Serra, A., Neeper, J. et al. A randomized controlled trial of differing doses of postcesarean enoxaparin thromboprophylaxis in obese women. J Perinatol 36, 95–99 (2016). https://doi.org/10.1038/jp.2015.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2015.130
This article is cited by
-
Evaluation of unmet clinical needs in prophylaxis and treatment of venous thromboembolism in at-risk patient groups: pregnancy, elderly and obese patients
Thrombosis Journal (2019)
-
Challenges of Anticoagulation Therapy in Pregnancy
Current Treatment Options in Cardiovascular Medicine (2017)