Abstract
Blunted nocturnal dipping in blood pressure (BP) is associated with increased cardiovascular disease (CVD) risk in middle-aged/older adults. The prevalence of blunted nocturnal BP dipping is higher in persons with obesity and diabetes, conditions that are also associated with elevated aortic stiffness and inflammation. Therefore, we hypothesized that elevated glycemia, inflammation and aortic stiffness would be inversely associated with the magnitude of nocturnal systolic BP dipping among middle-aged/older adults with obesity at high CVD risk. Twenty-four hour ambulatory BP monitoring, aortic stiffness (carotid–femoral pulse wave velocity, CF-PWV), hemoglobin A1c (HbA1c) and inflammation (C-reactive protein, CRP) were measured in 86 middle-aged/older adults with obesity and at least one other CVD risk factor (age 40–74 years; 34 male/52 female; body mass index=36.7±0.5 kg m−2; HbA1c=5.7±0.04%). In the entire cohort, CRP (β=0.40±0.20, P=0.04), but not HbA1c or CF-PWV was independently associated with systolic BP dipping percent (Model R2=0.07, P=0.12). In stratified (that is, presence or absence of prediabetes) multiple linear regression analysis, HbA1c (β=6.24±2.6, P=0.02) and CRP (β=0.57±0.2, P=0.01), but not CF-PWV (β=0.14± 2.6, P=0.74), were independently associated with systolic BP dipping percent (Model R2=0.32, P<0.01) in obese adults with prediabetes but were absent in obese adults without prediabetes (Model R2=0.01 P=0.95). However, nocturnal systolic BP dipping percent (P=0.65), CF-PWV (P=0.68) and CRP (P=0.59) were similar between participants with and without prediabetes. These data suggest that impaired long-term glycemic control and higher inflammation may contribute partly to blunted BP dipping in middle-aged/older adults with obesity-related prediabetes.
Similar content being viewed by others
Introduction
A decline in systolic blood pressure (BP) ⩾10% at night during sleep is a normal physiological phenomenon referred to as nocturnal BP ‘dipping’. A blunted decline in nocturnal systolic BP (that is, ‘non-dipping’) is associated with target organ damage such as increased left ventricular mass and central arterial stiffness1, 2, 3 and heightened cardiovascular disease (CVD) mortality, independent of average 24 h systolic BP.4 However, the mechanisms underlying blunted nocturnal systolic BP dipping remain unclear.
The nocturnal non-dipping BP pattern is more prevalent in obesity5 and the prevalence of non-dipping in individuals with diabetes may be as high as 30%.6 In addition, individuals with obesity are more likely to exhibit higher systemic inflammation compared with normal-weight adults.7, 8 This is important, because inflammation likely contributes to the development of CVD in obesity in part because of insulin resistance and vascular dysfunction including aortic stiffness,8, 9 a robust independent predictor of CVD risk in middle-aged/older adults.10, 11 Moreover, the combination of obesity and metabolic dysfunction results in the greatest risk for CVD and death, underscoring the importance of glycemic control for CVD risk management in obese adults with early stage type 2 diabetes (that is, prediabetes).12
Given that 86 million Americans have prediabetes,13 more than double the number with diagnosed type 2 diabetes,13, 14 it is important to identify modifiable risk factors to attenuate CVD risk in this group. However, whether the prevalence of blunted nocturnal systolic BP dipping is higher in obese adults in the presence or absence of prediabetes is unknown. Furthermore, the intermediary mechanisms that might contribute to abnormal systolic BP dipping in obesity-related prediabetes have not been explored.
The purpose of the study was to determine whether systemic inflammation, recent glycemic control and aortic stiffness were independently associated with nocturnal BP dipping in a cohort of middle-aged/older adults with obesity and at high CVD risk. We hypothesized that elevated glycemia (hemoglobin A1c, HbA1c), inflammation (C-reactive protein, CRP) and aortic stiffness (carotid–femoral pulse wave velocity, CF-PWV) would be inversely associated with the magnitude of nocturnal systolic BP dipping (dipping percent) among middle-aged/older adults with obesity. Second, we also hypothesized that the associations between HbA1c, CRP and CF-PWV would be selective to obese persons with prediabetes. This is clinically important, because this would provide evidence that impaired long-term glycemic control, inflammation and arterial stiffness may contribute to blunted nocturnal systolic BP dipping, which has been linked to subclinical and overt CVD1, 2, 4 in obesity.
Methods
Study design
Ninety-nine middle-aged/older adults were recruited but complete data were available in 86 of these individuals (34 male/52 female) for analyses. The analyses used baseline data in 86 middle-aged/older adults (age 40–75 years) with obesity who were recruited as part of a pharmacological weight loss study (Clinical Trials.gov study NCT01351753) who had 24 h ambulatory BP monitoring done at baseline. Participants were included in the study if they were obese, defined as having a body mass index (BMI) ⩾30 kg m−2 and had at least one additional CVD risk factor such as hypertension, hyperlipidemia, hyperglycemia or metabolic syndrome, or who were being treated for a CVD risk factor with anti-hypertensive medications or anti-hyperlipidemia medications. Participants were excluded if they had a history of smoking cessation within the past 3 months, had a diagnosed atherosclerotic event within the past 6 months, had congestive heart failure (WHO class III or IV), had renal impairment defined as serum creatinine ⩾1.4 mg dl−1 (females) or ⩾1.5 mg dl−1 (males), or if they were currently involved in any weight loss or formal vigorous exercise program. All women were postmenopausal of whom eight were on hormone replacement therapy: transdermal patch and removed the patch 24 h before the experimental visit (n=3); oral hormone replacement therapy and went off for 1 month before measurements (n=2); and intravaginal cream or tablets for postmenopausal symptoms (n=3). Fifteen participants were taking levothyroxine with doses ranging from 50 to 150 mcg per day and had normal TSH concentrations at time of study. The study was approved by the University of Iowa Institutional Review Board and all participants signed written informed consent.
Experimental visit
Participants reported to the University of Iowa Institute for Clinical and Translational Science Clinical Research Unit after an overnight 8 h fast. Height and weight were measured and a fasting venous blood sample was obtained. Following 15 min of supine rest, BP and aortic stiffness were measured (see below for details). Participants were fitted and started to wear a 24 h ambulatory BP monitor at the end of the visit and instructed to wear the monitor for 24 h. As a result of scheduling, in some instances participants wore the 24 h ambulatory BP monitor before the experimental visit.
Body mass index
Height and weight were measured with participants wearing a standard gown and no shoes and after participants emptied their bladder. Measurements were done using a stadiometer and a digital scale by a trained nurse. BMI was calculated by dividing weight (in kg) by height (in m2).
Twenty-four hour ambulatory BP
Ambulatory BP monitoring was performed for 24 h with a cuff on the non-dominant arm, (Spacelabs Healthcare, Inc., Snoqualmie, WA, USA). Participants were instructed to record their activities and sleep periods for the 24 h monitoring period. BP was measured every 30 min during the day from 0600 to 2300 h and nighttime (that is, ‘nocturnal’) BP was recorded every 60 min from 2300 to 0600 h. Daytime and nocturnal BPs were determined from these fixed times. Participants had to have at least 10 daytime readings and 5 nighttime readings and at least 80% successful readings of planned measurements over the 24 h. The percent (%) of nocturnal systolic BP dipping was calculated as:

Aortic stiffness
CF-PWV, the ‘reference standard’ measurement of aortic stiffness, was obtained non-invasively by applanation tonometry (Sphygmocor, AtCor Medical, Inc., NSW, Australia) as previously described.15, 16 Electrocardiogram-gated pulse waveforms of the carotid, femoral and radial arteries were recorded sequentially and PWV was calculated as path length (L) divided by time delay (t) between sites (PWV=L/t). L was calculated as distance from the suprasternal notch to femoral minus the distance from the suprasternal notch to the carotid artery. The peak of the R wave from the simultaneously recorded electrocardiogram was used as a timing marker. Within-subject coefficient of variation for CF-PWV in our laboratory is 2.1% for nine adults measured on two visits separated by 1 week.
Circulating factors
Circulating concentrations of fasting insulin, glucose, HbA1C, cholesterol and triglycerides were measured in venous blood sample by the University of Iowa Diagnostic Laboratory by standard techniques. CRP was measured by the University of Iowa Diagnostic Laboratory by a high-sensitivity turbidimetric method with a minimal detection level of 0.2 mg l−1. Prediabetes was defined as an HbA1C value ⩾5.7 and not being treated with any antidiabetic drugs. Homeostatic model of insulin resistance was calculated as follows: insulin (mU ml−1) × glucose (mg dl−1)/405.
Risk of obstructive sleep apnea
As nocturnal BP and metabolic health17 can be influenced by the presence of obstructive sleep apnea (OSA), we used the Berlin Survey to determine whether participants had high risk of OSA. This survey has been validated and used in other cohort studies. Briefly, participants were categorized as high or low risk for OSA based on their responses to questions regarding snoring, daytime sleepiness and CV risk factors as previously described.18 Complete data on likelihood of OSA at the time of vascular measurements and ambulatory BP monitoring were available in 42 participants.
Statistics
Normality of distribution for variables of interest was assessed using Shapiro–Wilk tests. Differences between adults with and without prediabetes were evaluated using unpaired t-tests or Wilcoxon rank-sum tests for non-normally distributed variables and χ2-tests for categorical variables. Univariate correlations of nocturnal systolic dipping percent and established CVD risk factors were assessed using Spearman’s correlations. Associations between aortic stiffness, circulating factors and nocturnal systolic BP dipping were evaluated using multivariable regression, first in the entire cohort and then stratified by pre-diabetes status because of the well-characterized effect of diabetes on nocturnal systolic BP dipping.19, 20 A third multivariable model was constructed to adjust for known modifiers of BP and nocturnal dipping. We also tested for associations between these factors and average nocturnal systolic BP because nocturnal BP is associated with CVD and non-CVD mortality. A residuals versus fitted plot was constructed for each significant predictor variable to assess the assumptions of linearity and homoscedasticity. A sensitivity analysis was performed on a subset of participants that excluded those with possible undiagnosed diabetes (HbA1c⩾6.5%) and CRP level >2 s.d. from the mean. Significance was set at P<0.05 and STATA version 14 (College Station, TX, USA) was used for all statistical analyses.
Results
Participants
Characteristics of the cohort of the eighty-six individuals with obesity and at least 1 CVD risk factor (34 male/52 female) used for analyses are listed in Table 1. Thirty-eight percent of all participants were taking renin–angiotensin–aldosterone system antagonists. However, the proportion of participants taking renin–angiotensin–aldosterone system antagonists was the same (38%) in participants with and without prediabetes (P=1.00).
Bivariate correlational and regression analyses in entire cohort
No traditional CVD risk factors significantly correlated with nocturnal systolic BP dipping percent in our cohort (P>0.13 for all). Among our hypothesized predictors, CRP (r=0.25, P=0.015) but not CF-PWV (r=0.11, P=0.29) or HbA1c (r=0.13, P=0.215) was correlated with nocturnal systolic BP dipping in the entire cohort. In a multivariable analysis including CRP, HbA1c and CF-PWV conducted in the entire cohort, only CRP was associated with nocturnal systolic BP dipping percent (β=0.40, P=0.04) (Table 2).
Participants stratified by the presence or absence of prediabetes
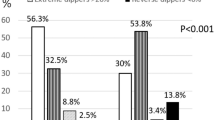
Of the 86 participants, 50 had prediabetes, defined as HbA1C⩾5.7% and not taking antidiabetic medication,21 and 36 did not have prediabetes (Table 3). Participants with prediabetes were slightly older than participants without prediabetes (P=0.01), but BMI did not differ by prediabetes status (P=0.59). Clinic, daytime and nocturnal BP and CF-PWV were similar in adults with and without prediabetes (all P>0.05, Table 3). Nocturnal systolic BP dipping percent was the same in adults with (−10.5±6.4%) and without (−9.9±6.1%) prediabetes (P=0.65, Table 3). Three participants with prediabetes and one participant without prediabetes were ‘risers’, that is, their nocturnal systolic BP was higher than their daytime systolic BP and their dipping was positive. Four participants with prediabetes and one participant without prediabetes were extreme dippers; their systolic BP dipped >20% at night. There was no difference in the proportion of participants with either pattern by prediabetes status (P>0.47 for both comparisons).
Fourteen participants without prediabetes and 15 participants with prediabetes had high risk of OSA (P=0.12 for difference between groups).
Bivariate correlational and regression analyses in participants with or without prediabetes
In individuals with prediabetes, CRP (r=0.34, P=0.015) and HbA1c (r=0.26, P=0.05), but not CF-PWV (r=0.09, P=0.51), were correlated with nocturnal systolic BP dipping percent in bivariate correlational analysis. In contrast, in individuals without prediabetes none of the hypothesized predictors correlated with nocturnal systolic BP dipping percent: CRP (r=−0.15, P=0.36), HbA1c (r=0.01, P=0.95), CF-PWV (r=−0.05, P=0.79). In the stratified unadjusted regression analysis with HbA1c, CRP and CF-PWV in the model, HbA1c (β=6.24, P=0.02) and CRP (β=0.57, P=0.01), but not CF-PWV (β=0.14, P=0.74), were independently associated with nocturnal systolic BP dipping percent in adults with prediabetes (Table 4a). These associations were absent in middle-aged/older adults without prediabetes (Table 4b). In a third stratified model in which we controlled for age, sex, average 24 h systolic BP, and anti-hypertensive and lipid-lowering medication usage, the associations between CRP (β=0.57, P=0.12) and HbA1c (β=7.35, P=0.19) with nocturnal systolic dipping percent remained significant (Table 4c).
To investigate whether indices of short-term glucose/insulin control or OSA were also associated with nocturnal systolic BP dipping percent, fasting insulin (β=0.32, P=0.612), fasting glucose (β=0.15, P=0.267) and fasting homeostatic model of insulin resistance (β= −1.5, P=0.571) were not associated with nocturnal systolic BP dipping percent in the whole cohort, with similar findings in stratified analyses (Supplementary Table 1). OSA status was not associated with nocturnal systolic BP dipping percent in the whole cohort (β=0.35±2.2, P=0.87) or in stratified analyses.
As we had three statistical outliers (>2 s.d. from the mean) for our CRP measurement, one individual with an extreme reverse dipping pattern (+15% compared with daytime systolic BP) and two individuals with HbA1c>6.5%, the cutoff for diabetes, we conducted a sensitivity analysis by performing the regression with these individuals omitted (n=80, 35 without prediabetes and 45 with prediabetes). Our results remained the same: HbA1c (β=9.87, P=0.027) and CRP (β=0.97, P=0.006) but not CF-PWV (β=0.02, P=0.96), were independently associated with nocturnal systolic BP dipping percent in adults with prediabetes (Model R2=0.26, P=0.006). These associations remained absent in adults without prediabetes, neither HbA1c (β=−2.38, P=0.41), CRP (β=0.07, P=0.78), nor CF-PWV (β=0.16, P=0.71) associated with nocturnal dipping percent (Model R2=0.03, P=0.82).
There was no association between clinic systolic BP and nocturnal systolic dipping percent (β=0.14±0.04, P=0.76). Finally, to test whether CRP, HbA1c and CF-PWV were also associated with average nocturnal systolic BP, in a multivariable model including age, sex, BMI, anti-hypertensive medication usage, CRP, HbA1c and CF-PWV, only CF-PWV was associated with average nocturnal systolic BP; β=1.97±0.8, P=0.01 in the whole cohort, driven by the prediabetes group in stratified analyses (Supplementary Table 2).
Discussion
The primary findings of the current study are that higher HbA1c and circulating CRP concentrations are independently associated with less nocturnal systolic BP dipping percent only in individuals with obesity and prediabetes even after adjusting for age, sex, average 24 h systolic BP and anti-hypertensive medication usage. There was no relation between systolic BP dipping percent with short-term indices of glycemia (fasting glucose) or insulin resistance (fasting insulin and homeostatic model of insulin resistance) among obese adults without prediabetes. These findings suggest that CVD risk conferred by impaired longer-term glycemic control (HbA1c) not only directly contributes to elevated arterial stiffening and daytime BP as previously described,22 but that elevated glycemia may also interfere with normal physiological nocturnal BP dipping in obesity. Furthermore, these results indicate that once middle-aged/older adults with obesity have prediabetes, small incremental increases in HbA1c may be associated with significantly more blunting of nocturnal systolic BP dipping compared with those without prediabetes. It reasons that even small improvements in HbA1c may improve nocturnal systolic BP dipping in middle-aged/older persons with obesity-related prediabetes. The study also supports the role of systemic inflammation as either a mediator of blunted nocturnal BP dipping or an identifier of overall higher risk among this sub-group of obese adults with prediabetes. Thus, whether reducing systemic inflammation would also attenuate blunted nocturnal systolic BP dipping in this group will require additional studies to test these hypotheses.
Blunted nocturnal systolic BP dipping is more common in persons with obesity5 and the prevalence of blunted nocturnal systolic BP dipping in type 2 diabetes is over 30%.6 Nighttime systolic BP is also predictive of CVD mortality in individuals with diabetes,23 but whether this is true for adults with prediabetes is unclear. Surprisingly, in the current cohort we found that the nocturnal BP dipping percent was not different between obese adults with and without prediabetes. However, among the obese persons with prediabetes defined as an HbA1C ⩾5.7%, HbA1C was independently associated with blunted nocturnal systolic BP dipping (β=7.35, P=0.010), suggesting that impaired long-term glycemic control may affect systolic BP dipping in obese persons with prediabetes. Consistent with this, biomarkers associated with short-term glucose control (for example, fasting glucose, insulin and HOMA) were not associated with nocturnal systolic BP dipping in our whole cohort or by prediabetes status. This suggests that longer-term and not short-term glycemic control potentially modulates nocturnal systolic BP dipping in obese adults with prediabetes. This finding is not entirely unexpected as short-term biomarkers are highly variable and may be affected by the patient’s recent dietary intake, physical activity or length of fasting.
Circulating CRP, a biomarker of systemic inflammation and CVD risk, is elevated in persons with obesity.24 In addition, higher CRP is associated with increased arterial stiffness in middle-aged/older adults25 that are both elevated in individuals with obesity.24 Anti-inflammatory therapy is associated with reductions in aortic stiffness and aortic inflammation supporting the idea that inflammation has a key role in aortic remodeling and stiffness.26 Arterial stiffness is associated with less nocturnal systolic BP dipping among adults with hypertension,1, 27 but before our study it was unknown whether higher inflammation and arterial stiffness could be potential intermediary mechanisms that modulate alterations in nocturnal BP dipping among older adults with obesity. Taken together, our results and others indicate that systemic inflammation among obese adults with prediabetes may be one intermediary mechanism for blunted nocturnal BP dipping, but prospective studies with follow-up or anti-inflammatory intervention studies will be needed to confirm this finding.
Because of the association of average nocturnal systolic BP and CVD risk, we investigated whether HBA1c, CRP and CF-PWV were associated with average nocturnal systolic BP itself (as opposed to just percent dipping). There was an independent association between aortic stiffness and average nocturnal systolic BP, independent of age and BMI. Given that aortic stiffness has been purported to precede incident hypertension, and aortic stiffness improves risk prediction for CVD events in models with traditional CVD risk factors, these results are not unprecedented.11, 28 There was no association between clinical systolic BP and nocturnal dipping in our study, underscoring the importance of monitoring 24 h BP to assess CVD risk. Our study suggests that higher aortic stiffness may contribute to overall CVD risk in part through its influence on nocturnal BP, but further studies are required to test this hypothesis.
A limitation of our study is the use of data collected at a single time point in a relatively small sample. This may have limited our ability to detect small to mid-sized effects. We included information regarding the likelihood of sleep apnea in our study, but we did not obtain this measurement on all participants. Therefore, we may not have captured the true prevalence of sleep apnea in our cohort. The cross-sectional design does not allow us to determine cause and effect between HbA1c or CRP with nocturnal systolic BP dipping in the cohort with prediabetes. Longitudinal data would be useful to assess how changes in HbA1c and CRP at multiple time points alter nocturnal systolic BP dipping profiles within the same person. We are unable to determine whether blunted nighttime systolic BP dipping is associated with mortality in obese persons with prediabetes; therefore, this should be examined in future investigations in prospective studies. Lastly, we did not record the timing of anti-hypertensive medication usage although most participants likely take the medications in the morning. Whether or not participants took medication immediately before bedtime may have affected their nocturnal BP. A strength of our study is the similarity of the well characterized cohort of persons with obesity at high CVD risk and multivariable stratified models adjusting for important confounders.
In conclusion, in middle-aged/older adults with obesity and at least one other CVD risk factor, higher HbA1c and CRP are independently associated with less nocturnal systolic BP dipping selectively among those persons with prediabetes (defined by elevated HbA1c). Prediabetes status itself did not affect the magnitude of nocturnal systolic BP dipping among obese adults. Our study has demonstrated that longer-term glycemic control and inflammation may contribute to CVD risk through their association with nocturnal BP dipping. Future studies are needed to determine if improvements in HbA1c and CRP in early stages of type 2 diabetes may improve normal physiological dipping of nighttime systolic BP and decrease CVD risk in this high-risk group.
References
Cicek Y, Durakoglugil ME, Kocaman SA, Cetin M, Erdogan T, Dogan S, Ugurlu Y, Canga A . Non-dipping pattern in untreated hypertensive patients is related to increased pulse wave velocity independent of raised nocturnal blood pressure. Blood Pressure 2013; 22: 34–38.
Cuspidi C, Sala C, Tadic M, Rescaldani M, Grassi G, Mancia G . Non-dipping pattern and subclinical cardiac damage in untreated hypertension: a systematic review and meta-analysis of echocardiographic studies. Am J Hypertens 2015; 28: 1392–1402.
O'Flynn AM, Madden JM, Russell AJ, Curtin RJ, Kearney PM . Isolated nocturnal hypertension and subclinical target organ damage: a systematic review of the literature. Hypertens Res 2015; 38: 570–575.
Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y . Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002; 20: 2183–2189.
Ben-Dov IZ, Bursztyn M . Ambulatory blood pressure monitoring in childhood and adult obesity. Curr Hypertens Rep 2009; 11: 133–142.
Fogari R, Zoppi A, Malamani GD, Lazzari P, Destro M, Corradi L . Ambulatory blood pressure monitoring in normotensive and hypertensive type 2 diabetes. Prevalence of impaired diurnal blood pressure patterns. Am J Hypertens 1993; 6: 1–7.
Debnath M, Agrawal S, Agrawal A, Dubey GP . Metaflammatory responses during obesity: pathomechanism and treatment. Obes Res Clin Pract 2016; 10: 103–113.
Isomaa B . A major health hazard: the metabolic syndrome. Life Sci 2003; 73: 2395–2411.
Park S, Lakatta EG . Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J 2012; 53: 258–261.
Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB . Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17635 subjects. J Am Coll Cardiol 2014; 63: 636–646.
Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ . Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121: 505–511.
Fan J, Song Y, Chen Y, Hui R, Zhang W . Combined effect of obesity and cardio-metabolic abnormality on the risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Int J Cardiol 2013; 168: 4761–4768.
Centers for Disease Control and Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. In: Services USDoHaH, ed. U.S. Department of Health and Human Services: Atlanta, GA; 2014.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015; 314: 1021–1029.
Lane AD, Yan H, Ranadive SM, Kappus RM, Sun P, Cook MD, Harvey I, Woods J, Wilund K, Fernhall B . Sex differences in ventricular-vascular coupling following endurance training. Eur J Appl Sci 2014; 114: 2597–2606.
Pierce GL, Zhu H, Darracott K, Edet I, Bhagatwala J, Huang Y, Dong Y . Arterial stiffness and pulse-pressure amplification in overweight/obese African-American adolescents: relation with higher systolic and pulse pressure. Am J Hyprtens 2013; 26: 20–26.
Shimizu I, Yoshida Y, Minamino T . A role for circadian clock in metabolic disease. Hypertens Res 2016; 39: 483–491.
Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP . Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Int Med 1999; 131: 485–491.
Ayala DE, Moya A, Crespo JJ, Castineira C, Dominguez-Sardina M, Gomara S, Sineiro E, Mojón A, Fontao MJ, Hermida RC . Hygia Project Investigators. Circadian pattern of ambulatory blood pressure in hypertensive patients with and without type 2 diabetes. Chronobiol Int 2013; 30: 99–115.
Cuspidi C, Vaccarella A, Leonetti G, Sala C . Ambulatory blood pressure and diabetes: targeting nondipping. Curr Diabetes Rev 2010; 6: 111–115.
American Diabetes Association. 2. Classification and diagnosis of diabetes. Diabetes Care 2016; 39 (Suppl 1): S13–S22.
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE . Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 2015; 17: 1180–1193.
Draman MS, Dolan E, van der Poel L, Tun TK, McDermott JH, Sreenan S, O'Brien E . The importance of night-time systolic blood pressure in diabetic patients: Dublin Outcome Study. J Hypertens 2015; 33: 1373–1377.
Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB . Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999; 282: 2131–2135.
Yasmin, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB . C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscl Thromb Vasc Biol 2004; 24: 969–974.
Wang S, Ma AQ, Song SW, Quan QH, Zhao XF, Zheng XH . Fish oil supplementation improves large arterial elasticity in overweight hypertensive patients. Eur J Clin Nutr 2008; 62: 1426–1431.
Castelpoggi CH, Pereira VS, Fiszman R, Cardoso CR, Muxfeldt ES, Salles GF . A blunted decrease in nocturnal blood pressure is independently associated with increased aortic stiffness in patients with resistant hypertension. Hypertens Res 2009; 32: 591–596.
Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF . Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012; 308: 875–881.
Acknowledgements
We thank Christine Sinkey and the University of Iowa Institute for Clinical and Translational Science nurses and staff for their assistance with this study. This study was supported by National Institutes of Health grants 5T32 HL007638, 5T32 HL007121, U54 TR001013, 5 KL2 RR24980-5, 2P01 HL014388, 1K01 AG047626, 1R21 AG043722 and American Heart Association grant 13SDG143400012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Lane-Cordova, A., Kalil, G., Wagner, C. et al. Hemoglobin A1c and C-reactive protein are independently associated with blunted nocturnal blood pressure dipping in obesity-related prediabetes. Hypertens Res 41, 33–38 (2018). https://doi.org/10.1038/hr.2017.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2017.82
Keywords
This article is cited by
-
Non-dipping pattern in early-stage diabetes: association with glycemic profile and hemodynamic parameters
Journal of Human Hypertension (2022)
-
Association of risk factors for atherosclerosis, including high-sensitivity C-reactive protein, with carotid intima-media thickness, plaque score, and pulse wave velocity in a male population
Hypertension Research (2020)
-
The relationship between Plasma Markers and Essential Hypertension in Middle-aged and Elderly Chinese Population: A Community Based Cross-sectional Study
Scientific Reports (2019)
-
Impaired nocturnal blood pressure dipping in patients with type 2 diabetes mellitus
Hypertension Research (2019)
-
Determinants of pulse pressure amplification in hypertensive and diabetic patients
Hypertension Research (2019)