Abstract
Circulating renin–angiotensin system (RAS) activation is maintained after renal function has deteriorated. The activation of the intrarenal RAS plays a critical role in the pathophysiology of chronic kidney disease (CKD), independently of the circulating RAS. However, the activation of intrarenal RAS and the chymase-dependent pathway after initiation of dialysis has not been clarified. We recruited 19 CKD patients (10 without dialysis and 9 with dialysis) who underwent a heminephrectomy. Circulating RAS was investigated before nephrectomy. The levels of intrarenal RAS components and chymase-positive cells were investigated using radioimmunoassay or immunoblot analysis on samples collected from the removed kidney. Renal damage was evaluated by the extent of tubulointerstitial fibrosis. No significant differences in circulating RAS between nondialysis and dialysis patients were found. However, intrarenal angiotensin II (AngII) and the extent of tubulointerstitial fibrosis in dialysis patients were significantly increased when compared with nondialysis patients. Prorenin and angiotensin-converting enzyme (ACE) levels were dramatically decreased in accordance with renal dysfunction. On the other hand, chymase-positive cells and AngII type 1 receptor (AT1R) expression was significantly increased in dialysis patients when compared with nondialysis patients. In multiple linear regression analyses, there were significant positive and negative relationships between the extent of interstitial fibrosis and angiotensinogen (β=0.45, P=0.042) and prorenin levels (β=−0.85, P<0.01), respectively. In summary, a decrease in prorenin and ACE expression and an increase in chymase, angiotensinogen and AT1R expression in the kidney may augment the intrarenal RAS activation and be associated with renal damage, even after initiation of dialysis.
Similar content being viewed by others
Introduction
The kidneys play a critical role in the filtration of water and waste products, erythropoiesis by means of erythropoietin generation, modulation of bone and mineral metabolism by means of 1α-hydroxylase activation and regulation of the circulating and intrarenal renin–angiotensin system (RAS). It is well known that erythropoietin generation and 1α-hydroxylase activation gradually decrease because of impaired renal function.
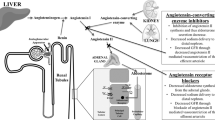
The circulating RAS has a circadian rhythm and plays an important role in blood pressure (BP) and sodium homeostasis.1, 2 In normal subjects, the circulating RAS is measurable by plasma renin activity (PRA) and is generally suppressed by volume overload and BP elevation. PRA is not completely suppressed in chronic renal failure patients and, as a result, hypertension and normal to high levels of PRA are maintained.3, 4 Moreover, it has been reported that PRA increases in patients on maintenance hemodialysis (HD) over an 8- to 10-year period.5 On the other hand, in renal transplant patients where the patients’ native kidneys were nephrectomized, BP was decreased and daily antihypertensive requirements were reduced.6 Furthermore, when a bilateral nephrectomy was performed, stable plasma concentrations at 5–10% (renin and angiotensins) and 25–30% (prorenin) of pre-nephrectomy levels were reached during a period of just 1 to 2 days after nephrectomy.7 It has further been reported that BP after bilateral nephrectomy is significantly lowered and that plasma renin concentration falls to undetectable levels, whereas the angiotensin II (AngII) receptor blocker loses its hypotensive effect in bilateral nephrectomized animals.8 These results indicate that the circulating RAS is determined by renin that is secreted from the juxtaglomerular apparatus in the kidneys and that the ability to secrete renin is maintained, even when renal function is completely deteriorated
It has been found that activation of the intrarenal RAS including aldosterone plays a critical role in the pathophysiology of chronic kidney disease (CKD) and hypertension, independent of the circulating RAS.2, 9, 10
We have shown that intrarenal RAS activation due to upregulation of intrarenal angiotensinogen (AGT) expression is induced in certain animal models11, 12, 13, 14 and that intrarenal RAS is activated in CKD patients, where urinary AGT excretion is used as a surrogate marker of intrarenal RAS activation.15, 16, 17 Previous studies have demonstrated that AngII causes hypoxia in the kidney by inducing structural microvasculature damage and fibrotic changes, leading to end-stage renal disease. Furthermore, AngII induces oxidative stress that in turn consumes nitric oxide and results in inefficient oxygen usage.18, 19 However, it is difficult to investigate the intrarenal RAS activation in animal models with end-stage renal disease, as these animals would need to be kept alive by dialysis and it is impossible to evaluate intrarenal RAS activation in dialysis patients using the measure of urinary AGT excretion, as these patients do not excrete urine.
Some potential alternative enzymes other than angiotensin-converting enzyme (ACE) can synthesize AngII from angiotensin I (AngI), and there is evidence suggesting that chymase derived from mast cells plays a critical role in AngII synthesis in cardiovascular tissues.20, 21, 22 Moreover, Akasu et al.23 reported that chymase-dependent AngII formation was more dominant than ACE-dependent AngII formation in aorta extracts of normotensive rats, whereas Murakami et al.24 discovered that intra-arterial infusion of [Pro11, D-Ala12]-AngI, which is inactive but yields AngII by a mechanism dependent on chymase and not ACE, induces renal vasoconstriction. These data support the potential contribution of a chymase-dependent pathway in cardiovascular tissues and activation of this pathway may lead to the progression of cardiovascular injuries.
Thus, the purpose of this study was to clarify whether intrarenal RAS and chymase in dialysis patients whose renal function has been completely abolished is activated and identify which RAS components are associated with intrarenal RAS activation.
Methods
Patients
This study was approved by the ethics committee of Hamamatsu University School of Medicine (No. 25–92) and was conducted in accordance with the guidelines provided by the Declaration of Helsinki. All patients enrolled in this study provided written informed consent. We recruited 19 CKD patients with renal dysfunction (10 patients without dialysis and 9 patients with dialysis) who were admitted to our hospital and who underwent heminephrectomy between September 2013 and September 2015, regardless of their antihypertensive medication.
Study protocols
In patients on HD, vital signs such as height, dry weight, systolic and diastolic BPs and heart rate were measured the day before surgery, after which blood samples were drawn and HD conducted subsequently. Both patients on peritoneal dialysis and those not on dialysis also underwent the aforementioned procedures preoperatively. These procedures were performed 1.21±0.98 days before nephrectomy.
Patients were asked to rest in the supine position for at least 15 min before blood sample collection that was scheduled at 0900 h. Thereafter, blood samples were centrifuged at 3000 r.p.m. for 10 min at 4 °C and stored at −80 °C until further assay, as per a previous report.16 Kidney samples were collected immediately after nephrectomy and prepared as described in the subsequent sections.
Measurement of renal function and circulating RAS concentrations
Serum creatinine concentrations were measured in the clinical laboratory at our hospital. The estimated glomerular filtration rate was calculated using the Japanese estimated glomerular filtration rate equation.25 Plasma renin activity and plasma AngII levels for circulating RAS were determined by radioimmunoassay (SRL, Tokyo, Japan).
Evaluation of tubulointerstitial lesions
A small part of the resected kidney was fixed in formalin and embedded in paraffin. Tissue sections (3 μm) were stained with Masson’s trichrome for histopathological evaluation of tubulointerstitial lesions. As the extent of tubulointerstitial fibrosis reflects the severity of renal damage,26 the percentages of tubulointerstitial fibrosis were evaluated in microscopic fields observed at × 100 magnification. Ten microscopic fields were evaluated for each patient using a point-counting method and mean values were calculated. All quantitative analyses were performed by a blinded operator to avoid bias.
Intrarenal AngII contents
Intrarenal AngII levels were measured by radioimmunoassay using an AngII antibody (SRL) according to the manufacturer’s instructions. Intrarenal AngII concentrations were normalized against the weight of the resected kidney fragment.
Immunoblot and immunohistochemical analysis of intrarenal RAS
In order to evaluate the expression levels of intrarenal RAS components, immunoblot analysis of renin and prorenin, (pro)renin receptor ((P)RR)), AGT, ACE and AngII type 1 receptor (AT1R) was conducted as previously described.11, 12, 13, 14, 26 The primary antibodies were a rabbit anti-renin/ prorenin antibody (generously provided by Dr T Senbonmatsu of Saitama Medical University, Moroyama, Japan; and Dr T Inagami of Vanderbilt University, Nashville, TN, USA), a rabbit anti-(P)RR antibody (Abcam, Tokyo, Japan), a mouse anti-AGT antibody (IBL, Takasaki, Japan), a goat anti-ACE antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a rabbit anti-AT1R antibody (Santa Cruz Biotechnology). A mouse anti-β-actin antibody (Santa Cruz Biotechnology) was also used. The accumulation of these proteins as determined by immunoblot analysis was normalized against that of β-actin. The densitometric ratios were calculated relative to nondialysis patients for each RAS component.
Immunostaining for (P)RR, AGT and AT1R in kidney sections was performed using the EnVision+Dual Link System-HRP (Dakocytomation, Glostrup, Denmark), whereas immunostaining for ACE was performed using a Histofine kit (Nichirei-Bioscience, Tokyo, Japan) as previously described.11, 12, 13, 14, 26 The antibodies used for immunoblot analysis were also used for immunostaining (rabbit anti-(P)RR antibody, mouse anti-AGT antibody, rabbit anti-AT1R antibody and goat anti-ACE antibody). Immunostaining for chymase was also performed similarly. The primary antibody was a mouse anti-chymase antibody (Abnova, Taipei, Taiwan). Chymase-positive cells were counted in microscopic fields at × 200 magnification. Ten microscopic fields were evaluated for each patient and mean values were calculated. All quantitative analyses were performed by a blinded operator to avoid bias. Sections incubated without primary antibodies were used as a control. As the anti-renin/prorenin antibody reacts with both renin and prorenin, it is impossible to distinguish immunostaining between the two; thus, this antibody was not used in the present study.
Statistical analysis
Results are expressed as the mean±s.d. Dry weight was used as body weight for the purpose of analyses in patients on HD. PRA and intrarenal AngII did not show a normal distribution and logarithmic transformation was therefore applied in this case. Statistical significance between nondialysis and dialysis patients was determined using Student’s t-test for unpaired samples. The significance of differences in causes of nephrectomy, past history or comorbidity and antihypertensive usage was examined using the χ2 test. Correlations between the extent of interstitial fibrosis and other parameters were evaluated using Pearson’s product-moment correlation coefficient. Multiple linear regression analyses of the extent of interstitial fibrosis were adjusted for age, sex, body weight, systolic BP, plasma AngII and the levels of intensity of intrarenal RAS components or chymase-positive cells. A P-value of <0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software package (Version 20, SPSS, Chicago, IL, USA).
Results
Patient characteristics
Patient characteristics are presented in Table 1. When nephrectomized patients were recruited into this study, the renal function of nine patients was <15 ml min−1 1.73 m−2 and all of them were dialysis patients; eight patients required HD and one required peritoneal dialysis. The mean dialysis period was 110.2±66.2 months.
Comparison between nondialysis and dialysis patients
The results of our comparison between nondialysis and dialysis patients are presented in Table 2. Although causes of nephrectomy, past history or comorbidity and the use of antihypertensives did not differ between the two groups, systolic BP in dialysis patients was significantly higher than that in nondialysis patients. No significant differences in circulating RAS expression levels (PRA and plasma AngII) were found. However, there were significant differences in intrarenal AngII expression levels. In addition, the extent of tubulointerstitial fibrosis in dialysis patients was significantly more severe than that observed in nondialysis patients.
Intrarenal RAS protein expression levels and regions
Immunoblotting was performed to investigate intrarenal RAS protein expression levels, as presented in Figure 1. Significant differences in (P)RR were not found when comparing nondialysis and dialysis patients. Prorenin was dramatically decreased according to renal dysfunction and there were significant differences between nondialysis and dialysis patients. On the other hand, although there were no significant differences between nondialysis and dialysis patients, renin level was slightly increased in dialysis patients. ACE level was also dramatically decreased according to renal dysfunction and significant differences were found between nondialysis and dialysis patients. AT1R expression levels in dialysis patients were significantly increased when compared with those in nondialysis patients. No significant differences in AGT expression levels were observed when comparing nondialysis and dialysis patients. However, AGT expression levels in dialysis patients tended to be higher than those observed in nondialysis patients (dialysis patients; 1.27±0.29 vs. nondialysis patients; 1.00±0.30; P=0.069).
Immunoblot of intrarenal renin–angiotensin system (RAS) components for nondialysis and dialysis patients. Representative protein expression levels of intrarenal RAS components for nondialysis and dialysis patients by immunoblot are shown. Filled bars indicate expression levels of nondialysis patients and dotted bars indicate those of dialysis patients. Numbers under the bands of immunoblot analysis indicate estimated glomerular filtration rate and numbers in parenthesis refer to the period (months) after which dialysis was introduced for each patient. The accumulation of these proteins as determined by immunoblot analysis is normalized against that of β-actin and the densitometric ratios are calculated relative to nondialysis patients for each RAS component. Asterisk refers to statistical difference of P<0.01 between nondialysis and dialysis patients. ACE, angiotensin-converting enzyme; AGT, angiotensinogen; AT1R, angiotensin II type 1 receptor; HD, hemodialysis; (P)RR, (pro)renin receptor.
Immunostaining for intrarenal RAS components and chymase was performed in order to identify expression regions and levels (Figure 2). Although levels of immunostaining for (P)RR were found in the proximal and distal tubular cells as well as the collecting ducts and connecting tubular cells in nondialysis patients, expression levels became weaker in dialysis patients. On the other hand, immunostaining results for small vessels did not significantly differ among patients, and infiltrated cells that stained positive for (P)RR were prominent in dialysis patients when compared with nondialysis patients. Immunostaining for AGT was abundant in the proximal tubules in nondialysis patients but decreased in dialysis patients. However, some tubular lumens were filled with AGT-positive materials in dialysis patients. Levels of immunostaining for ACE were abundant in the brush border of the proximal tubules in nondialysis patients and dramatically decreased in dialysis patients. Notably, ACE expression in patients who had been receiving dialysis for an extended period of time was almost undetectable. Infiltrated cells that were supposed to be mast cells were positive for chymase, and the number of chymase-positive cells in dialysis patients was significantly increased than in nondialysis patients (dialysis patients; 36.5±19.4 cells/field vs. nondialysis patients; 9.8±8.2 cells per field; P<0.01). Weak immunostaining for AT1R in the proximal and distal tubules was observed in nondialysis patients; however, immunostaining levels became much weaker in accordance with renal dysfunction. On the other hand, immunostaining levels for small vessels were intense and not significantly different between nondialysis and dialysis patients.
Immunohistochemistry of intrarenal renin–angiotensin system (RAS) components and chymase for nondialysis and dialysis patients. (Pro)renin receptor ((P)RR): Although the levels of immunostaining for (P)RR are found in the proximal and distal tubular cells as well as the collecting ducts and connecting tubular cells in nondialysis patients, the expression levels become weaker in dialysis patients. On the other hand, immunostaining results for small vessels do not significantly differ, and infiltrated cells positive for (P)RR are prominent in dialysis patients when compared with nondialysis patients. Angiotensinogen (AGT): Immunostaining for AGT is abundant in the proximal tubules in nondialysis patients, and decreased in dialysis patients. On the other hand, some tubular lumens are filled with AGT-positive materials in dialysis patients. Angiotensin-converting enzyme (ACE): Levels of immunostaining for ACE are abundant in the brush border of the proximal tubules of nondialysis patients and dramatically decreased in dialysis patients. Notably, ACE expression in patients receiving dialysis for an extended period is almost undetectable. Chymase: Infiltrated cells that are supposed to be mast cells are positive for chymase, and chymase-positive cells in dialysis patients are significantly increased compared with nondialysis patients. Angiotensin II type 1 receptor (AT1R): Weak immunostaining for AT1R in the proximal and distal tubules is found in nondialysis patients. However, immunostaining levels become much weaker in accordance with renal dysfunction. On the other hand, immunostaining levels for small vessels are intense and not significantly different between nondialysis and dialysis patients. The symbols number signs (#), asterisks (*), circles (○) and arrows (→) indicate the vessels, the collecting ducts and connecting tubules, proximal tubules and infiltrating cells positive for (P)RR, respectively. Numbers under the figures indicate estimated glomerular filtration rate and numbers in parenthesis refer to the period (months) after which dialysis was introduced for each patient. Original magnification × 200. HD, hemodialysis. A full color version of this figure is available at the Hypertension Research journal online.
Relationships between the extent of tubulointerstitial fibrosis and other parameters
We evaluated the correlation between the extent of tubulointerstitial fibrosis and other parameters, including BP, circulating and intrarenal RAS components and chymase (Table 3). The extent of tubulointerstitial fibrosis was significantly and positively correlated with systolic BP, whereas no significant correlation was found with the circulating RAS. On the other hand, the extent of tubulointerstitial fibrosis was significantly and positively correlated with chymase-positive cells, the intensity of AT1R and intrarenal AngII levels. Furthermore, although no significant relationships were found, the extent of tubulointerstitial fibrosis tended to be correlated with the intensity of AGT. Meanwhile, the extent of tubulointerstitial fibrosis was significantly and negatively correlated with the intensities of both prorenin and ACE.
Multiple linear regression analyses for measuring the extent of interstitial fibrosis
In order to evaluate the relationship between the extent of interstitial fibrosis and the contribution of intrarenal RAS components and chymase, we performed multiple linear regression analyses after adjusting for age, sex, body weight, systolic BP and plasma AngII levels (Table 4). Age, sex and body weight were selected as variables, as these parameters are commonly used when performing multiple linear regression analyses. In addition, both systolic BP and plasma AngII—the main component of the circulating RAS—were adjusted for multiple linear regression analyses, as they may be associated with renal damage. There were significant positive and negative relationships between the extent of interstitial fibrosis and AGT and prorenin intensity, respectively. Although chymase-positive cells, AT1R intensity and intrarenal AngII levels or ACE intensity tended to be positively or negatively associated with the extent of interstitial fibrosis, no significant relationships were found. On the other hand, no relationships at all were identified between the extent of interstitial fibrosis and renin or (P)RR intensity.
Discussion
We have previously shown that expression of intrarenal AGT activates the RAS and that this activation leads to renal damage in certain animal models of CKD that do not require dialysis,11, 12, 13, 14 as well as in CKD patients with a full range of renal function (with the exception of dialysis patients).15, 16, 17 Furthermore, it has been shown that intrarenal AngII may cause intrarenal AGT upregulation,27, 28 resulting in a cycle of intrarenal RAS activation. Concerning AT1R expression and the binding affinity of AngII, it has been shown that both AT1R mRNA and protein expression levels are increased in a remnant kidney model and that stretch or relaxation increases specific 125I AngII binding in cultured rat mesangial cells.29, 30, 31 In addition, we have recently found that intrarenal AGT and AT1R proteins are increased and the amplitude of the oscillations of these proteins is augmented, inducing renal damage in a model of chronic progressive glomerulonephritis.32 Considering the lack of evidence of expression levels of AGT and AT1R in dialysis patients compared with those in nondialysis patients, the major finding of this study is that intrarenal AGT and AT1R are augmented and that this upregulation leads to renal damage such as tubulointerstitial fibrosis and is associated with renal dysfunction in dialysis patients.
The levels of AGT measured by immunoblotting are inconsistent with the levels in proximal tubules observed upon immunohistochemistry for AGT. However, we believe that AGT in proximal tubular cells is derived from AGT that is produced in the liver and filtered from the impaired glomerular capillaries and AGT that is produced in the kidney by intrarenal AngII.33, 34 When renal damage deteriorates, and proximal tubular cells are diffusely and severely damaged, it is impossible to reabsorb and produce AGT in proximal tubular cells. As a result, AGT is rarely detected in proximal tubules by using immunohistochemistry. However, as some tubular lumens were found to be filled with AGT-positive materials in this study, it is possible that a large amount of AGT is filtered from damaged glomeruli and flows through the tubular lumens, and therefore AngII is produced in the tubular lumen by RAS components in the kidney and RAS-independent components such as chymase. The above-mentioned concepts require the presence of intratubular flow in dialysis patients. Some previous reports indicated that urinary volume was increased when tolvaptan was administered to HD and peritoneal dialysis patients.35, 36 This finding supports the idea that a certain amount of intratubular flow is preserved even in anuric dialysis patients.
Whereas staining of AT1R in the kidney is observed in all small arteries and arterioles including the afferent and efferent arterioles, the thick ascending limb epithelium and the proximal tubular epithelium and mesangial cells, the most intense staining is observed in the arterial and arteriolar smooth muscle among them.37 In fact, immunostaining of AT1R was remarkable for all arteries and arterioles in this study and similar levels were observed in both dialysis and nondialysis patients. Meanwhile, weak immunostaining for AT1R in the proximal and distal tubules was observed in nondialysis patients and these levels became much weaker in accordance with renal dysfunction. Contrary to the results of the immunostaining, immunoblot expression levels of AT1R in dialysis patients were significantly increased when compared with those in nondialysis patients. It is possible that structures other than small arteries and arterioles have insignificant effects and that the relative percentage increment of small arteries and arterioles due to the atrophy of structures, with the exception of vessels, may influence the increase in expression levels of AT1R in the kidney.
The expression level of prorenin was significantly decreased in accordance with renal damage. Prorenin is a precursor of renin and contains a prosegment that conceals the enzymatic cleft and obstructs access of AGT to the active site of renin. When the prosegment is removed by a processing enzyme, renin is generated and activates the RAS by proteolytic activation.38 (P)RR is a specific receptor for renin and prorenin and was identified as a component of the RAS by Nguyen et al.39 When prorenin binds to (P)RR, it gains renin activity mediated by conformational changes, leading to unfolding of the prosegment from the enzymatic cleft and nonproteolytic enzymatic activation.38 It has been reported that nonproteolytic activation can be induced by exposure to low pH (with an optimum pH of 3.3) and cold.40, 41 If these conditions are not present, proteolytic activation is more likely to occur than nonproteolytic activation. Thus, the decrease in prorenin and the increase of renin in accordance with progression of renal dysfunction observed in this study may reflect proteolytic activation of prorenin. Total expression levels of (P)RR by immunoblot were the same in nondialysis and dialysis patients, and these results are in line with our previous findings.17, 26
ACE is located on the endothelial cells of many vascular beds and on the membranes of various other cell types, including the brush border membranes of the proximal tubules.42 Metzger et al.43 reported that the localization of ACE within the kidney differs across species, and that the kidneys of normal human subjects predominantly express ACE in the brush border of the proximal tubular segments. As our results show, the regions of ACE expression identified by immunohistochemistry were limited to the brush border of the proximal tubules. Moreover, levels of ACE expression were dramatically decreased in accordance with renal dysfunction when measured by immunohistochemistry as well as immunoblot. On the other hand, chymase-positive cell counts were significantly increased in dialysis patients compared with nondialysis patients. These results suggest that a chymase-dependent pathway may contribute to the generation of AngII in place of ACE and renal damage according to renal dysfunction.
In summary, our results suggest that a decrease in prorenin and ACE expression and an increase in chymase, AGT and AT1R expression in the kidney may augment the intrarenal RAS and be associated with renal damage, even after initiation of dialysis.
References
Kala R, Fyhrquist F, Eisalo A . Diurnal variation of plasma angiotensin II in man. Scand J Clin Lab Invest 1973; 31: 363–365.
Kobori H, Nangaku M, Navar LG, Nishiyama A . The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59: 251–287.
Blankestijn PJ, Ligtenberg G, Klein IH, Koomans HA . Sympathetic overactivity in renal failure controlled by ACE inhibition: clinical significance. Nephrol Dial Transplant 2000; 15: 755–758.
Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ . Sympathetic hyperactivity in chronic kidney disease: pathogenesis, clinical relevance, and treatment. Kidney Int 2004; 65: 1568–1576.
Kimura G, Takahashi N, Kawano Y, Inenaga T, Inoue T, Nakamura S, Inoue T, Matsuoka H, Omae T . Plasma renin activity in hemodialyzed patients during long-term follow-up. Am J Kidney Dis 1995; 25: 589–592.
Power RE, Calleary JG, Hickey DP . Pre-transplant bilateral native nephrectomy for medically refractory hypertension. Ir Med J 2001; 94: 214–216.
Krop M, de Bruyn JH, Derkx FH, Danser AH . Renin and prorenin disappearance in humans post-nephrectomy: evidence for binding? Front Biosci 2008; 1: 3931–3939.
Bonnet JM, Boivin R, Bernard N, Sassard J . Extrarenal renin-angiotensin systems are unable to maintain blood pressure in sheep. Clin Exp Pharmacol Physiol 2000; 27: 684–689.
Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H . Regulation of intrarenal angiotensin II in hypertension. Hypertension 2002; 39: 316–322.
Sato A . The necessity and effectiveness of mineralocorticoid receptor antagonist in the treatment of diabetic nephropathy. Hypertens Res 2015; 38: 367–374.
Ohashi N, Yamamoto T, Huang Y, Misaki T, Fukasawa H, Suzuki H, Togawa A, Suzuki S, Fujigaki Y, Nakagawa T, Nakamura Y, Suzuki F, Kitagawa M, Hishida A . Intrarenal RAS activity and urinary angiotensinogen excretion in anti-thymocyte serum nephritis rats. Am J Physiol Renal Physiol 2008; 295: F1512–F1518.
Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H . Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol 2009; 36: 750–755.
Urushihara M, Ohashi N, Miyata K, Satou R, Acres OW, Kobori H . Addition of angiotensin II type 1 receptor blocker to CCR2 antagonist markedly attenuates crescentic glomerulonephritis. Hypertension 2011; 57: 586–593.
Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H . Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol 2008; 35: 922–927.
Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A . Urinary angiotensinogen as a marker of intrarenal angiotensin II activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol 2007; 18: 1558–1565.
Kobori H, Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Yamamoto T . Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens 2008; 2: 349–354.
Isobe S, Ohashi N, Fujikura T, Tsuji T, Sakao Y, Yasuda H, Kato A, Miyajima H, Fujigaki Y . Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol 2015; 19: 231–239.
Manotham K, Ongvilawan B, Urusopone P, Chetsurakarn S, Tanamai J, Limkuansuwan P, Tungsanga K, Eiam-Ong S . Angiotensin II receptor blocker partially ameliorated intrarenal hypoxia in chronic kidney disease patients: a pre-/post-study. Intern Med J 2012; 42: e33–e37.
Manotham K, Tanaka T, Matsumoto M, Ohse T, Miyata T, Inagi R, Kurokawa K, Fujita T, Nangaku M . Evidence of tubular hypoxia in the early phase in the remnant kidney model. J Am Soc Nephrol 2004; 15: 1277–1288.
Urata H, Ganten D . Cardiac angiotensin II formation: the angiotensin-I converting enzyme and human chymase. Eur Heart J 1993; 14 (Suppl I): 177–182.
Miyazaki M, Takai S . Role of chymase on vascular proliferation. J Renin Angiotensin Aldosterone Syst 2000; 1: 23–26.
Miyazaki M, Takai S . Local angiotensin II-generating system in vascular tissues: the roles of chymase. Hypertens Res 2001; 24: 189–193.
Akasu M, Urata H, Kinoshita A, Sasaguri M, Ideishi M, Arakawa K . Differences in tissue angiotensin II-forming pathways by species and organs in vitro. Hypertension 1998; 32: 514–520.
Murakami M, Matsuda H, Kubota E, Wakino S, Honda M, Hayashi K, Saruta T . Role of angiotensin II generated by angiotensin converting enzyme-independent pathways in canine kidney. Kidney Int Suppl 1997; 63: S132–S135.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992.
Huang Y, Yamamoto T, Misaki T, Suzuki H, Togawa A, Ohashi N, Fukasawa H, Fujigaki Y, Ichihara A, Nishiyama A, Senbonmatsu T, Ikegaya N, Hishida A . Enhanced intrarenal receptor-mediated prorenin activation in chronic progressive anti-thymocyte serum nephritis rats on high salt intake. Am J Physiol Renal Physiol 2012; 303: F130–F138.
Gonzalez-Villalobos RA, Seth DM, Satou R, Horton H, Ohashi N, Miyata K, Katsurada A, Tran DV, Kobori H, Navar LG . Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol 2008; 295: F772–F779.
Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Katsurada A, Navar LG, Kobori H . Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol 2008; 295: F283–F289.
Bautista R, Sánchez A, Hernández J, Oyekan A, Escalante B . Angiotensin II type AT2 receptor mRNA expression and renal vasodilatation are increased in renal failure. Hypertension 2001; 38: 669–673.
Cao Z, Bonnet F, Candido R, Nesteroff SP, Burns WC, Kawachi H, Shimizu F, Carey RM, De Gasparo M, Cooper ME . Angiotensin type 2 receptor antagonism confers renal protection in a rat model of progressive renal injury. J Am Soc Nephrol 2002; 13: 1773–1787.
Becker BN, Yasuda T, Kondo S, Vaikunth S, Homma T, Harris RC . Mechanical stretch/relaxation stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Exp Nephrol 1998; 6: 57–66.
Isobe S, Ohashi N, Ishigaki S, Tsuji T, Sakao Y, Kato A, Miyajima H, Fujigaki Y, Nishiyama A, Yasuda H . Augmented circadian rhythm of the intrarenal renin-angiotensin systems in anti-thymocyte serum nephritis rats. Hypertens Res 2016; 39: 312–320.
Matsusaka T, Niimura F, Shimizu A, Pastan I, Saito A, Kobori H, Nishiyama A, Ichikawa I . Liver angiotensinogen is the primary source of renal angiotensin II. J Am Soc Nephrol 2012; 23: 1181–1189.
Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG . Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol 2007; 293: F938–F945.
Mori T, Oba I, Koizumi K, Kodama M, Shimanuki M, Tanno M, Chida M, Saito M, Kiyomoto H, Miyazaki M, Ogawa S, Sato H, Ito S . Beneficial role of tolvaptan in the control of body fluids without reductions in residual renal function in patients undergoing peritoneal dialysis. Adv Perit Dial 2013; 29: 33–37.
Iwahori T, Esaki M, Hinoue H, Esaki S, Esaki Y . Tolvaptan increases urine and ultrafiltration volume for patients with oliguria undergoing peritoneal dialysis. Clin Exp Nephrol 2014; 18: 655–661.
Paxton WG, Runge M, Horaist C, Cohen C, Alexander RW, Bernstein KE . Immunohistochemical localization of rat angiotensin II AT1 receptor. Am J Physiol 1993; 264: F989–F995.
Danser AH, Deinum J . Renin, prorenin and the putative (pro)renin receptor. Hypertension 2005; 46: 1069–1076.
Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD . Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 2002; 109: 1417–1427.
Pitarresi TM, Rubattu S, Heinrikson R, Sealey JE . Reversible cryoactivation of recombinant human prorenin. J Biol Chem 1992; 267: 11753–11759.
Suzuki F, Hayakawa M, Nakagawa T, Nasir UM, Ebihara A, Iwasawa A, Ishida Y, Nakamura Y, Murakami K . Human prorenin has ‘gate and handle’ regions for its non-proteolytic activation. J Biol Chem 2003; 278: 22217–22222.
Schulz WW, Hagler HK, Buja LM, Erdös EG . Ultrastructural localization of angiotensin I-converting enzyme (EC 3.4.15.1) and neutral metalloendopeptidase (EC 3.4.24.11) in the proximal tubule of the human kidney. Lab Invest 1988; 59: 789–797.
Metzger R, Bohle RM, Pauls K, Eichner G, Alhenc-Gelas F, Danilov SM, Franke FE . Angiotensin-converting enzyme in non-neoplastic kidney diseases. Kidney Int 1999; 56: 1442–1454.
Acknowledgements
We acknowledge Dr Daisuka Motoyama, Dr Takayuki Sugiyama, Dr Masao Nagata, Dr Atsushi Otsuka, Dr Yasuo Ishii and Dr Hiroshi Furuse (Urology, Hamamatsu University School of Medicine) for performing the heminephrectomies and providing the kidney samples for this study. This study was supported by grants from the Young Investigator Research Projects of Hamamatsu University School of Medicine in 2013 and 2015 (awarded to Naro Ohashi), in 2013 and 2015 (awarded to Shinsuke Isobe) and in 2014 and 2015 (awarded to Sayaka Ishigaki).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ohashi, N., Isobe, S., Ishigaki, S. et al. Intrarenal renin–angiotensin system activity is augmented after initiation of dialysis. Hypertens Res 40, 364–370 (2017). https://doi.org/10.1038/hr.2016.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.143
Keywords
This article is cited by
-
Clinical and molecular correlation defines activity of physiological pathways in life-sustaining kidney xenotransplantation
Nature Communications (2023)
-
ACEi/ARBs associate with lower incidence of gastrointestinal bleeding in peritoneal dialysis patients
Clinical and Experimental Nephrology (2022)
-
Circadian rhythm of the intrarenal renin–angiotensin system is caused by glomerular filtration of liver-derived angiotensinogen depending on glomerular capillary pressure in adriamycin nephropathy rats
Hypertension Research (2021)
-
Possible benefits of exogenous melatonin for individuals on dialysis: a narrative review on potential mechanisms and clinical implications
Naunyn-Schmiedeberg's Archives of Pharmacology (2021)
-
Greater reductions in plasma aldosterone with aliskiren in hypertensive patients with higher soluble (Pro)renin receptor level
Hypertension Research (2018)