Abstract
Our aim was to assess the discrepancy in the blood pressure amplification (BPA) value defined as the aortic-to-brachial increase in systolic BP (SBP) between invasive and noninvasive brachial cuff-based methods. In 45 patients undergoing cardiac catheterization, BP in the brachial artery and ascending aorta were measured with an invasive catheter and a brachial cuff-based oscillometric device. To calculate aortic SBP, brachial waveforms were calibrated by the brachial systolic and diastolic BP (DBP) (C1 calibration) or by the brachial mean BP and DBP (C2 calibration). C1 calibration underestimated aortic SBP (−17.7 mm Hg (95% confidence interval: −21.9 to −13.5)), whereas C2 calibration generated an approximately accurate aortic SBP (1.8 mm Hg (−2.4 to 5.9)). Regarding brachial SBP, noninvasively measured values were markedly underestimated (22.2 mm Hg (−26.4 to −18.0)), resulting in a slightly low BPA value in C1 calibration (11.9±6.3 mm Hg) and a paradoxical negative BPA value in C2 calibration (−7.6±6.7 mm Hg). Multiple linear regression analysis showed that the cuff-catheter difference of BPA was positively correlated with the cuff-catheter difference of brachial SBP in both calibrations (C1 calibration: β=0.51; C2 calibration: β=0.50; both P<0.01). Although noninvasively measured BPA was associated with invasively measured BPA only in C1 calibration (r=0.33, P=0.03), when using invasively measured brachial SBP instead of a cuff-based measurement, the BPA was well associated with invasively measured BPA in both calibrations (C1 calibration: r=0.57; C2 calibration: r=0.52; both P<0.001). In conclusion, there was a trade-off in accuracy between brachial cuff-based noninvasive aortic SBP and BPA because of the inherent inaccuracies in the cuff-based method. This finding should be fully considered in establishing standardized reference values for aortic BP.
Similar content being viewed by others
Introduction
Direct measurement of intra-arterial blood pressure (BP) demonstrates a physiological increase in systolic BP (SBP) from the central to peripheral artery,1, 2 generally referred to as BP amplification (BPA). BPA is mainly caused by differences in vessel stiffness and wave reflection,3 and is potentially useful for cardiovascular risk prediction4, 5 and detection of high amplification typically observed in isolated systolic hypertension among the young.6, 7 However, noninvasive and easy-to-perform measurements for brachial and aortic SBP are required for the use of BPA values in daily clinical practice.
Brachial BP is measured noninvasively with a cuff-based oscillometric method worldwide, but the noninvasively measured brachial SBP value is not accurate and is considerably lower than the invasively measured value8, 9; this difference is certain to influence the accuracy of BPA estimation. It is unrealistic to modify this systematic underestimation of brachial SBP given the overwhelming diffusion and methodological limitations of oscillometric devices.
Several noninvasive methods for estimating aortic BP have been developed recently.10 The SphygmoCor device (AtCor, Sydney, NSW, Australia), a radial artery applanation tonometer, has been most commonly used in epidemiological studies.11 Recently developed brachial cuff-based oscillometric methods are highly available because of their easy-to-use, operator-independent methodologies12, 13 and allow the widespread use of central aortic BP in various situations. Each approach has its own strengths and limitations, but the common problem is that the accuracy of the estimated aortic BP is compromised mainly because of an error in the brachial BP measurement used for calibration, which has been widely debated.14, 15, 16, 17 Whenever a cuff-based oscillometric method is used for brachial BP estimation, aortic BP indices are sure to be inaccurate.
Thus, both brachial and aortic BP measurement errors affect the accuracy of BPA estimation by a noninvasive method; however, few studies have assessed the BPA value using both noninvasive and invasive techniques. Ding et al.18 investigated the SphygmoCor and Omron HEM-9000AI (Omron Healthcare, Kyoto, Japan) devices for the estimation of BPA compared with invasive measurement. Although the two devices had similar precision in the estimation of pressure amplification, there was a considerable difference in absolute values. In addition, a systematic analysis also raised the issue of compatibility because noninvasive aortic BP and BPA estimation is device and technique dependent, and results obtained with one technique are not applicable to other devices.11 However, no other aortic BP recording devices have been assessed for their accuracy in BPA estimation. Here we measured brachial and aortic BP invasively and noninvasively using the Mobil-O-Graph brachial cuff-based oscillometric device (IEM GmbH, Stolberg, Germany), to investigate the discrepancy in BPA values between invasive and noninvasive methods.
Methods
Subjects
We enrolled patients who underwent elective coronary angiography at our institution between June 2014 and September 2014. We excluded patients with hemodynamically significant valvular heart disease, left ventricular outflow tract obstruction, persistent cardiac arrhythmia or a >5 mm Hg difference between the left and right brachial SBP.16 Of the 51 patients who were screened, 6 patients with arrhythmias during evaluation or with invalid aortic BP readings provided by Mobil-O-Graph were excluded. Accordingly, the present analysis included 45 participants. The institutional ethics committee approved the study protocol and written informed consent was obtained from each participant.
Hypertension was defined as brachial SBP⩾140 mm Hg or DBP⩾90 mm Hg, or prescription of an antihypertensive drug. The presence of diabetes mellitus was defined as a fasting blood glucose level ⩾126 mg dl−1 or the use of a hypoglycemic agent or insulin. Chronic kidney disease was defined as an estimated glomerular filtration rate <60 ml min−1 per 1.73 m2. Significant coronary artery disease was defined as the presence of >50% stenosis in one or more major coronary arteries.
Measurement of hemodynamic indices
All hemodynamic measurements were performed with the patient in the supine position immediately before coronary angiography. A 4- or 5-Fr introducer sheath 25 cm in length (Radifocus, Terumo Medical, Tokyo, Japan) was placed via a radial approach (right radial approach: 51%, left radial approach: 49%) and the intra-arterial pressure at the tip of the sheath was measured as the brachial BP. Immediately after the measurement, a fluid-filled catheter was positioned in the ascending aorta and aortic SBP and DBP were measured. The aortic and brachial BPs were simultaneously measured using the Mobil-O-Graph on the contralateral arm. Only high-quality recordings of the brachial waveforms by an automated device assessment and by visual inspection were used. Invasive measurement was performed for at least 10 s, to derive an average value using a clinical polygraph (RMC-4000; Nihon Koden, Tokyo, Japan). The pressure transducer was zeroed to the atmosphere before each measurement and maintained at the level of the mid-axillary line during the examination. The natural frequency of this system was at least 20 Hz and the damping coefficient was at least 0.3. For noninvasive measurements, either the brachial SBP and DBP (C1) or the brachial mean BP (MBP) and DBP (C2) measured by the Mobil-O-Graph were used for calibration, because the calibration methods affect the accuracy of aortic BP estimation.12 BPA was calculated by subtracting aortic from brachial SBP.
Statistical analysis
All data were analyzed using STATA version 13.0 (StataCorp, College Station, TX, USA). All continuous values were expressed as the mean±s.d. and categorical variables were reported as percentages. Discrepancies between invasively and noninvasively assessed BP indices were examined using the paired samples t-test and Bland–Altman plots. Correlations among BPA values measured with invasive and noninvasive methods were analyzed using Pearson’s method. Linear regression analysis was performed to investigate the determinants of BPA measurement error. The difference between invasively and noninvasively measured SBP and the SBP level were included in the multivariate regression model (model 1) based on the findings of a previous report.18 Possible confounders among baseline characteristics with P-values <0.15 were added in model 2. All P-values were two-tailed. P-values <0.05 were considered statistically significant.
Results
Participants and demographic baselines
The 45 patients in the study population included 39 men (86.7%), 35 patients with coronary artery disease (77.8%) and 31 hypertensive patients (68.9%). The mean age was 65.9±9.0 years and the mean body mass index was 24.2±2.9 kg m−2 (Table 1). Thirty-three patients were prescribed BP-lowering agents.
Invasive BP and its amplification
Invasive measurements provided a considerably higher value of brachial SBP compared with aortic SBP (164.1±28.6 vs 147.7±26.2 mm Hg, P<0.001; Table 2), leading to positive values for BPA (16.4±10.5). The DBP value was almost the same between the aortic root and the brachial artery, resulting in a higher value of brachial pulse pressure (PP) compared with aortic PP (88.0±25.5 vs. 72.7±22.2 mm Hg, P<0.001).
Brachial cuff-based BP and its amplification
In the cuff-based noninvasive measurements, brachial SBP was 141.9±20.5 mm Hg and was underestimated by 22.2 mm Hg (95% confidence interval (CI) −26.4 to −18.0 mm Hg) compared with invasive brachial SBP. Aortic SBP by the C1 and C2 calibration methods was 130.0±18.7 and 149.5±22.0 mm Hg, respectively. The former was much lower (−17.7 mm Hg (95% CI −21.9 to −13.5 mm Hg), P<0.001) and the latter was slightly higher (mean difference: 1.8 mm Hg (95% CI −2.4 to 5.9)) than the invasive aortic SBP. This difference in accuracy along with the lower brachial SBP caused a slightly lower BPA in the C1 calibration (11.9±6.3 mm Hg; mean difference: −4.5 mm Hg (95% CI −7.6 to −1.4)) and a markedly underestimated, paradoxically negative BPA in the C2 calibration (−7.6±6.7 mm Hg; mean difference: −23.9 mm Hg (95% CI −27.5 to −20.4 mm Hg)). Noninvasive brachial DBP and aortic DBP in both calibrations were overestimated to the same extent; thus, PP was consistently underestimated regardless of measurement. Among PPs attained, aortic PP with the C1 calibration was lower and with the C2 calibration was higher than brachial PP (brachial PP: 52.2 mm Hg; aortic PP with the C1 calibration: 39.2 mm Hg; aortic PP with the C2 calibration: 58.0 mm Hg; Table 2).
Determinants of the measurement error of noninvasive BPA
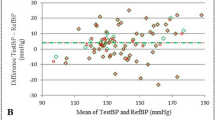
BPA by the C1 calibration method, but not the C2 calibration method, exhibited a significant correlation with invasively assessed BPA (C1: r=0.33, P=0.03; C2: r=0.10, P=0.50; Figures 1a and b). A significant inverse trend was noted between the difference and average of BPA values derived from invasive and noninvasive methods in each calibration method (Figures 1c and d). To investigate the determinants of the measurement error of BPA, we performed linear regression analysis for cuff-catheter differences in BPA. In univariate analysis, the cuff-catheter difference in brachial SBP significantly correlated with the cuff-catheter difference of BPA in both calibration methods (C1 calibration: r=0.36, P=0.02; C2 calibration: r=0.43, P=0.003; Supplementary Figure S1). These correlations remained significant in the multivariate analysis after adjustment for the brachial SBP level and possible confounders (Table 3). To eliminate the effect of the measurement error in brachial SBP, we calculated BPA using noninvasive aortic SBP and invasive brachial SBP instead of cuff-based methods. As a result, BPA with either calibration showed a good correlation with the invasively assessed BPA (C1 calibration: r=0.57; C2 calibration: r=0.52; all P<0.001; Supplementary Figure S2).
(a) Scatterplot of noninvasively measured BPA using the C1 calibration method (BPAcal1) versus invasively measured BPA (BPAinv). The C1 calibration method used the brachial SBP and DBP to calculate aortic BP. (b) Scatterplot of noninvasively measured BPA with the C2 calibration method (BPAcal2) versus invasively measured BPA. The C2 calibration method used the brachial mean BP and DBP to calculate aortic BP. (c) Bland–Altman plot of the data presented in 1A. (d) Bland–Altman plot of the data presented in 1B. For the scatterplots, the dotted lines indicate the lines of identity. The line of regression is drawn as a solid line only when statistically significant. For the Bland–Altman plots, the lines at the mean value (solid lines) and plus and minus two s.d. (dotted lines) are included. BPA, blood pressure amplification.
Discussion
To the best of our knowledge, this is the first study to investigate the discrepancy between BPA values estimated with a brachial cuff-based device and an invasive catheter. Calibration methods considerably affected the accuracy of aortic SBP estimation and the more accurate value of aortic SBP calibrated with brachial MBP and DBP resulted in a paradoxical negative value of BPA, as observed in a previous study12. In the present study, we used invasive measurement of brachial BP to reveal that the substantial underestimation of brachial SBP derived by the cuff-based oscillometric method was the main cause of this discrepancy.
This study showed that a brachial cuff-based oscillometric device, Mobil-O-Graph, underestimated brachial SBP by 22.2 mm Hg and overestimated DBP by 13.6 mm Hg, leading to the underestimation of PP. The high frequency of hypertensive patients and high BP values potentially affected the accuracy of the estimation. Ding et al.18 reported a comparably larger underestimation of brachial SBP (18.2 mm Hg) using the Omron HEM-9000AI in a population relatively similar to that of the present study in terms of the incidence of hypertension (69.7%) and the baseline BP profiles. However, several reports also demonstrated consistent results with underestimation of brachial SBP and overestimation of brachial DBP regarding the use of an oscillometric method.8, 9, 18, 19 The essential fact is that oscillometric devices yield a brachial BP with systematic errors regardless of the patient’s background factors and devices. Despite the consistent measurement error, its ability to predict future cardiovascular disease is firmly established; thus, cuff-derived brachial BP is essential for the definition and management of hypertension now and probably in the future. However, this measurement error raises another problem when aortic BP is estimated noninvasively.
We found that the Mobil-O-Graph underestimated aortic SBP by 17.7 mm Hg when brachial SBP and DBP were used for calibration. This finding is consistent with a previous validation study of this device.12 The effect of calibration error on the noninvasive estimation of aortic SBP has been widely debated.14, 15 Although the SphygmoCor device has been widely used as a suitable comparative standard, this device can derive accurate aortic SBP when calibrated with invasively measured brachial BP.2 This device considerably underestimates aortic SBP when calibrated with noninvasively measured brachial SBP and DBP.20, 21 Herbert et al.22 chose noninvasively estimated aortic BP calibrated with cuff brachial BP to establish reference values for central BP and its amplification, allowing the underestimation of both noninvasive aortic and brachial SBP. This underestimation might lead to an accurate noninvasive estimation of SBP amplification, similar to our findings obtained from aortic SBP calibrated with brachial SBP and DBP. However, there is a systematic underestimation of noninvasive aortic SBP as well as cuff-based brachial SBP, and it no longer seems possible to change this in clinical practice.
Our findings and those of a previous study suggest that the Mobil-O-Graph yields accurate aortic SBP values with brachial MBP and DBP calibration (abbreviated as the C2 calibration method) but not with brachial SBP and DBP calibration (abbreviated as the C1 calibration method).12 A very recent meta-analysis investigating the accuracy of commercial devices also revealed that the C2 calibration provides more accurate estimation of aortic SBP than the C1 calibration.23 In addition, some studies using the Mobil-O-Graph reported the superiority of aortic SBP with the C2 calibration compared with the C1 calibration in the discriminatory power for cardiac structural abnormalities24, 25 and the prognostic ability for mortality.26 The accuracy and clinical effectiveness of estimated aortic SBP supports the superiority of MBP over SBP for calibration at least when estimating aortic SBP by the Mobil-O-Graph.
Despite the accuracy, the aortic SBP with the C2 calibration resulted in a paradoxical negative value of BPA. A systematic meta-analysis reported that BPA values measured with the HEM-9000AI and Arteriograph (TensioMed, Budapest, Hungary) devices were negative, with weighed mean values of −1.14 and −2.57 mm Hg, respectively.11 These findings are inconsistent with the general understanding that brachial SBP is higher than aortic SBP. We showed that the underestimation of cuff-based brachial SBP was the main cause of this inconsistency. This systematic underestimation of brachial SBP may also result in a lack of difference between cuff-based brachial SBP and invasively measured aortic SBP.27 Ironically, accurate aortic SBP estimation with HEM-9000AI,18 Arteriograph28 and Mobil-O-Graph devices, when calibrated with brachial MBP and SBP, leads to the cancellation of BPA. These findings suggest that establishing reference values for aortic BP and amplification is an intractable problem.
In addition, the BPA obtained with the C2 calibration method did not correlate with invasively assessed BPA in contrast to the BPA obtained with the C1 calibration method. The BPA, and the difference between the brachial and aortic SBP, might be more accurately assessed by the C1 calibration method, because the brachial cuff-based SBP is directly transferred to the aortic SBP estimation. In contrast, the brachial SBP is not directly transferred to the aortic SBP estimation in the C2 calibration method, although brachial SBP is used to calculate the BPA value. The accumulation of independent errors in brachial SBP and aortic SBP measurements might lead to the unreliable BPA value in the C2 calibration method. We found that the cuff-catheter difference in brachial SBP was an independent determinant of the BPA measurement error in both the C1 and C2 calibration methods. When the BPA was calculated using noninvasive aortic SBP and invasive brachial SBP instead of cuff-based brachial SBP, to eliminate the effect of brachial SBP measurement error, the BPA in the C2 calibration method showed a good correlation with the invasively measured BPA, suggesting that brachial SBP measurement error is a substantial cause of the unreliability of the BPA value in the C2 calibration method. The C2 calibration seems to be superior at estimating aortic SBP, but the C1 calibration method might be superior when assessing BPA with this device. Further studies investigating the clinical effectiveness of BPA for each calibration method are needed to confirm this superiority.
There are some limitations to the present study. First, as many patients had hypertension, high mean values of BP were recorded in this study, which might have led to the relatively large measurement errors in brachial SBP and DBP compared with previous reports. However, similar results regarding the BPA value were obtained after excluding the hypertensive patients (data not shown), suggesting that our finding is applicable regardless of the presence of hypertension. Second, the accuracy of the invasive BP measurement using a fluid-filled catheter is affected by damping and attenuation, resulting in either overestimation or underestimation of BP. The use of a high-fidelity pressure transducer would increase the accuracy of the recorded pressure waveform. Finally, brachial and aortic BPs were estimated with a brachial cuff-based oscillometric device. Although this device has been validated with acceptable results,12 it remains unclear whether our results are applicable to other devices. In addition, aortic BP estimation is device dependent and a device-specific reference value for diagnosis is required.11, 18 However, the fundamental properties discussed here are probably consistent among devices. Moreover, the aortic BP value can be obtained in an easy-to-use, operator-independent manner with this device and its availability might bring central hemodynamics into daily clinical use.
In conclusion, this study demonstrated a trade-off in accuracy between brachial-cuff based noninvasive aortic SBP and BPA. This inherent problem in the cuff-based method should be fully considered in establishing standardized reference values for aortic BP.
References
Kroeker EJ, Wood EH . Comparison of simultaneously recorded central and peripheral arterial pressure pulses during rest, exercise and tilted position in man. Circ Res 1955; 3: 623–632.
Pauca AL, O'Rourke MF, Kon ND . Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001; 38: 932–937.
Avolio AP, Van Bortel LM, Boutouyrie P, Cockcroft JR, McEniery CM, Protogerou AD, Roman MJ, Safar ME, Segers P, Smulyan H . Role of pulse pressure amplification in arterial hypertension: experts’ opinion and review of the data. Hypertension 2009; 54: 375–383.
McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, Rowe CV, Cockcroft JR, Wilkinson IB . Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension 2008; 51: 1476–1482.
Chirinos JA, Kips JG, Jacobs DR Jr., Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P . Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol 2012; 60: 2170–2177.
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M, Wood DA . 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34: 2159–2219.
O'Rourke MF, Adji A . Guidelines on guidelines: focus on isolated systolic hypertension in youth. J Hypertens 2013; 31: 649–654.
Kobayashi H, Kinou M, Takazawa K . Correlation between the brachial blood pressure values obtained using the cuff method and the central blood pressure values obtained invasively. Intern Med 2013; 52: 1675–1680.
Ding FH, Fan WX, Zhang RY, Zhang Q, Li Y, Wang JG . Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am J Hypertens 2011; 24: 1306–1311.
McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB . Central blood pressure: current evidence and clinical importance. Eur Heart J 2014; 35: 1719–1725.
Narayan O, Casan J, Szarski M, Dart AM, Meredith IT, Cameron JD . Estimation of central aortic blood pressure: a systematic meta-analysis of available techniques. J Hypertens 2014; 32: 1727–1740.
Weber T, Wassertheurer S, Rammer M, Maurer E, Hametner B, Mayer CC, Kropf J, Eber B . Validation of a brachial cuff-based method for estimating central systolic blood pressure. Hypertension 2011; 58: 825–832.
Sugawara R, Horinaka S, Yagi H, Ishimura K, Honda T . Central blood pressure estimation by using N-point moving average method in the brachial pulse wave. Hypertens Res 2015; 38: 336–341.
Adji A, O'Rourke MF . Brachial artery tonometry and the Popeye phenomenon: explanation of anomalies in generating central from upper limb pressure waveforms. J Hypertens 2012; 30: 1540–1551.
Shih YT, Cheng HM, Sung SH, Hu WC, Chen CH . Quantification of the calibration error in the transfer function-derived central aortic blood pressures. Am J Hypertens 2011; 24: 1312–1317.
Martin JS, Borges AR, Christy JBT, Beck DT . Considerations for SphygmoCor radial artery pulse wave analysis: side selection and peripheral arterial blood pressure calibration. Hypertens Res 2015; 38: 675–683.
Salvi P, Grillo A, Parati G . Noninvasive estimation of central blood pressure and analysis of pulse waves by applanation tonometry. Hypertens Res 2015; 38: 646–648.
Ding FH, Li Y, Zhang RY, Zhang Q, Wang JG . Comparison of the SphygmoCor and Omron devices in the estimation of pressure amplification against the invasive catheter measurement. J Hypertens 2013; 31: 86–93.
Ochiai H, Miyazaki N, Miyata T, Mitake A, Tochikubo O, Ishii M . Assessment of the accuracy of indirect blood pressure measurements. Jpn Heart J 1997; 38: 393–407.
Cloud GC, Rajkumar C, Kooner J, Cooke J, Bulpitt CJ . Estimation of central aortic pressure by SphygmoCor requires intra-arterial peripheral pressures. Clin Sci (Lond) 2003; 105: 219–225.
Smulyan H, Siddiqui DS, Carlson RJ, London GM, Safar ME . Clinical utility of aortic pulses and pressures calculated from applanated radial-artery pulses. Hypertension 2003; 42: 150–155.
Herbert A, Cruickshank JK, Laurent S, Boutouyrie P . Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J 2014; 35: 3122–3133.
Papaioannou TG, Karageorgopoulou TD, Sergentanis TN, Protogerou AD, Psaltopoulou T, Sharman JE, Weber T, Blacher J, Daskalopoulou SS, Wassertheurer S, Khir AW, Vlachopoulos C, Stergiopulos N, Stefanadis C, Nichols WW, Tousoulis D . Accuracy of commercial devices and methods for noninvasive estimation of aortic systolic blood pressure a systematic review and meta-analysis of invasive validation studies. J Hypertens 2016; 34: 1237–1248.
Protogerou AD, Argyris AA, Papaioannou TG, Kollias GE, Konstantonis GD, Nasothimiou E, Achimastos A, Blacher J, Safar ME, Sfikakis PP . Left-ventricular hypertrophy is associated better with 24-h aortic pressure than 24-h brachial pressure in hypertensive patients: the SAFAR study. J Hypertens 2014; 32: 1805–1814.
Negishi K, Yang H, Wang Y, Nolan MT, Negishi T, Pathan F, Marwick TH, Sharman JE . Importance of calibration method in central blood pressure for cardiac structural abnormalities. Am J Hypertens 2016; 29: 1070–1076.
Wassertheurer S, Baumann M . Assessment of systolic aortic pressure and its association to all cause mortality critically depends on waveform calibration. J Hypertens 2015; 33: 1884–1888. discussion 1889.
Davies JI, Band MM, Pringle S, Ogston S, Struthers AD . Peripheral blood pressure measurement is as good as applanation tonometry at predicting ascending aortic blood pressure. J Hypertens 2003; 21: 571–576.
Horvath IG, Nemeth A, Lenkey Z, Alessandri N, Tufano F, Kis P, Gaszner B, Cziraki A . Invasive validation of a new oscillometric device (Arteriograph) for measuring augmentation index, central blood pressure and aortic pulse wave velocity. J Hypertens 2010; 28: 2068–2075.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
YK received lecture fees from Daiichi-Sankyo (Tokyo, Japan), Takeda Pharmaceutical (Osaka, Japan), Bayer Yakuhin (Osaka, Japan) and Boehringer Ingelheim (Ingelheim, Germany). YK received research grants from Boehringer Ingelheim (Ingelheim, Germany), Pfizer (New York, USA), Otsuka Pharmaceutical (Tokyo, Japan), Takeda Pharmaceutical (Osaka, Japan), Mitsubishi Tanabe Pharma (Osaka, Japan), Sumitomo Dainippon Pharma (Osaka, Japan), Astellas Pharma (Tokyo, Japan), St Jude Medical (St Paul, USA), Abbott Vascular Japan (Tokyo, Japan) and Daiichi-Sankyo (Tokyo, Japan). All other authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Rights and permissions
About this article
Cite this article
Nakagomi, A., Okada, S., Shoji, T. et al. Comparison of invasive and brachial cuff-based noninvasive measurements for the assessment of blood pressure amplification. Hypertens Res 40, 237–242 (2017). https://doi.org/10.1038/hr.2016.132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2016.132
Keywords
This article is cited by
-
A systematic review of invasive, high-fidelity pressure studies documenting the amplification of blood pressure from the aorta to the brachial and radial arteries
Journal of Clinical Monitoring and Computing (2021)
-
Association Between Central-Peripheral Blood Pressure Amplification and Structural and Functional Cardiac Properties in Children, Adolescents, and Adults: Impact of the Amplification Parameter, Recording System and Calibration Scheme
High Blood Pressure & Cardiovascular Prevention (2021)
-
Impact of Methodological and Calibration Approach on the Association of Central and Peripheral Systolic Blood Pressure with Cardiac Structure and Function in Children, Adolescents and Adults
High Blood Pressure & Cardiovascular Prevention (2019)
-
Validity of the augmentation index and pulse pressure amplification as determined by the SphygmoCor XCEL device: a comparison with invasive measurements
Hypertension Research (2018)
-
Systolic blood pressure amplification and waveform calibration
Hypertension Research (2017)