Abstract
Purpose:
To determine the effect of antibodies against recombinant human acid α-glucosidase (rhGAA) on treatment efficacy and safety, and to test whether the GAA genotype is involved in antibody formation.
Methods:
We used enzyme-linked immunosorbent assay (ELISA) to determine anti-rhGAA antibody titers at baseline and at 6, 12, and 36 months of rhGAA treatment. We measured the capacity of antibodies to neutralize rhGAA enzymatic activity or cellular uptake and the effects on infusion-associated reactions (IARs), muscle strength, and pulmonary function.
Results:
Of 73 patients, 45 developed antibodies. Maximal titers were high (≥1:31,250) in 22% of patients, intermediate (1:1,250–1:31,250) in 40%, and none or low (0–1:1,250) in 38%. The common IVS1/delex18 GAA genotype was absent only in the high-titer group. The height of the titer positively correlated with the occurrence and number of IARs (P ≤ 0.001). On the group level, antibody titers did not correlate with treatment efficacy. Eight patients (11%) developed very high maximal titers (≥156,250), but only one patient showed high sustained neutralizing antibodies that probably interfered with treatment efficacy.
Conclusions:
In adults with Pompe disease, antibody formation does not interfere with rhGAA efficacy in the majority of patients, is associated with IARs, and may be attenuated by the IVS1/delex18 GAA genotype.
Genet Med 19 1, 90–97.
Similar content being viewed by others
Introduction
Enzyme replacement therapy (ERT) is available for various lysosomal storage disorders, including Pompe disease (OMIM 232300), and for all these diseases antibody formation against ERT has been reported.1,2,3,4,5,6,7,8,9,10,11,12 Pompe disease is an autosomal recessive myopathy caused by deficiency of the lysosomal enzyme acid α-glucosidase (GAA) due to mutations in the GAA gene.13,14 In patients with the classic infantile phenotype, GAA activity is totally deficient; without therapy, patients die within the first year of life. In these patients, high sustained neutralizing antibody titers can interfere with ERT efficacy, particularly in cross-reactive immunologic material–negative patients who do not express any endogenous GAA protein.2,15 Children and adults with partial enzyme deficiency suffer from progressive limb-girdle muscular weakness and weakness of the respiratory muscles. When left untreated, most patients become wheelchair- and ventilator-dependent.16,17,18,19 Antibody formation is also suspected to potentially interfere with ERT in adult Pompe patients, but to date it is unknown how frequent and how serious this may be. Studies so far include four case reports on the likely interference of anti–recombinant human acid α-glucosidase (rhGAA) antibodies with ERT in adult patients20,21 and a short (18-month) follow-up of 59 patients in the Late Onset Treatment Study.4 Because the rate of disease progression is slow in adult Pompe patients,19 longer follow-up studies are required to assess the possible effects of antibodies on the clinical course. Here, we investigated anti-rhGAA antibody formation in a cohort of 73 adult Pompe patients during a period of 3 years of ERT. We measured their neutralizing effect on GAA enzymatic activity and cellular uptake and their impact on clinical outcome.
Materials and Methods
Patients and study design
This study is part of an ongoing, single-center, prospective, open-label study. All 73 patients received 20 mg/kg alglucosidase alfa intravenously every other week. The study protocol and inclusion criteria are described in more detail elsewhere.22 Additional inclusion criteria for this study were start of treatment between January 2005 and July 2010, and the availability of blood samples just before start of ERT and during ERT at 6, 12, and approximately 36 months. No pregnant patients participated in this study. Data were collected from 1 January 2005 through 1 January 2013. The institutional review board approved the study protocol, and all patients provided written informed consent.
Antibodies and neutralizing effects
Blood samples were drawn just before the infusions or, for patients receiving treatment at home, on a day when patients did not receive ERT. Some blood samples were not available at exactly 36 months ERT. In those cases, a sample taken between 24 and 42 months was used. Antibody titers were determined in plasma or serum using ELISA with fivefold dilution series.15 Experiments were performed in duplicate and repeated two to four times for high titers. The highest titer measured was used for analysis. Control sera and uncoated ELISA plates were used to determine background values, which ranged between 0 and 1:250. Antibody titers were determined per patient for all four time points (just before start of ERT and during ERT at 6, 12, and approximately 36 months) on the same plate. On each plate, a positive control (i.e., rabbit antiserum) and a negative control (serum from a healthy person) were included. The interassay coefficient of variation was 5%; the intra-assay coefficient of variation was 3%. For six patients, the ELISA results obtained during the Late Onset Treatment Study were used.4,23 For four of these, 6- and 12-month sera were retested with the ELISA developed at Erasmus MC with similar results. The neutralizing effects of antibodies were determined by addition of alglucosidase alfa and patient serum to the medium of GAA-deficient fibroblasts, followed by measurement of GAA activity in cells and medium.15 The experiment was performed in duplicate and repeated two to three times. GAA was expressed as a percentage of the activity present in medium containing fetal calf serum measured directly after addition of alglucosidase alfa to the medium.
Infusion-associated reactions and clinical outcome measures
Infusion-associated reactions (IARs) were recorded during alglucosidase alfa infusions by blinded investigators. Only IARs clinically judged to have a possible, probable, or definite relationship with the infusion were included. Skeletal muscle strength and pulmonary function tests were performed every 3 to 6 months before the start of ERT and every 3 months thereafter. Skeletal muscle strength was assessed using manual muscle testing according to the Medical Research Council (MRC) grading scale. A sum score was calculated by combining scores of the most affected muscles and was expressed as a percentage of the maximum possible score.22 Forced vital capacity (FVC) was measured in the upright and supine positions. Results were expressed as a percentage of predicted normal values.24,25
Statistical analysis
To calculate differences across the three antibody titer groups, the chi-squared trend test for nominal data and Kruskal-Wallis test for continuous and ordinal data were used. Patients were divided into three groups based on antibody titer to increase the power of statistical analysis. For estimating the effect of treatment, the treatment course was compared with the expected natural course based on the pretreatment data. For 58 of 73 patients, pretreatment data were available with a median follow-up of 16 months (range, 3–55 months). For all 73 patients, ERT treatment data were available with a median follow-up of 40 months (range, 24–42 months). To determine whether antibody formation influences treatment outcome, the following analyses were performed. For the total group, the effect of ERT on outcome measures was calculated using repeated measurements analysis for linear mixed effects models.26 This analysis corrects for the missing pretreatment data of 15 patients assuming that the data are missing at random. Comparison of these 15 patients with the remaining 58 patients showed no significant differences with respect to distribution among antibody titer groups, age at start of ERT, and disease duration (Supplementary Table S1 online). Because the evolution of each outcome measure before and after the start of ERT could be potentially nonlinear, we used as specification the natural cubic splines for both the fixed- and random-effects parts of the model. Linear evolutions were assumed for the period before ERT. For the period during ERT, a spline with boundary knots placed at 0 (=start of ERT) and 3.5 years of ERT and internal knots at 1.5 years were used. The model’s assumptions were checked using residuals plots. Treatment responses at 3 years of treatment were compared for the three antibody titer groups by adding an interaction term to the linear mixed effect models. Analyses were performed in SPSS for Windows (version 21; SPSS, Chicago, IL) and in R version 3.1.1 (2014-07-10) using package nlme (version 3.1–117). P<0.05 (two-sided) was considered statistically significant.
Results
Patients
General characteristics of the 73 patients at the start of ERT are shown in Table 1 . Treatment was started at a median age of 52 years (range, 26–74 years) after a median disease duration of 8 years (range, 0.1–32.2 years). Seventy-two patients received ERT during the entire study follow-up. One patient stopped ERT at 33 months, as reported.20 The median treatment duration at the last ELISA measurement was 35 months (range, 24–41 months).
Anti-rhGAA antibodies
Forty-six patients (63%) developed antibody titers to alglucosidase alfa above the background titer of 1:250. Titers varied widely between patients. For the entire group, the highest geometric mean titer of 1:1,337 was reached at 6 months after the start of ERT and declined thereafter to 1:275 at 36 months after ERT ( Figure 1a , bold line). Patients were divided into three groups according to their highest titer observed during the study period: 16 patients (22%) developed high titers (≥1:31,250), 29 patients (40%) developed intermediate titers (1:1250 to <1:31,250), and 28 patients (38%) developed no or low titers (0 to <1:1250) ( Figure 1b ). Antibody titer cutoffs were based on data derived from pediatric cases.1,2,15 Over 36 months of ERT, the intermediate- and high-titer groups showed mean trends similar to those of the total group ( Figure 1a ). Within the groups, antibody titer courses varied among patients. Three courses after 12 months of ERT were observed: a decreasing course, a stabilizing course, and an increasing course (Supplementary Figure S1 online). The majority of patients (97%) showed either stabilizing or decreasing antibody titer courses. Only two patients—one in the intermediate-titer group and one in the high-titer group—showed an increasing titer course. No significant differences were observed between patients with different titer courses with respect to age of disease onset, age at diagnosis, or GAA genotype (Supplementary Table S2 online).
Maximal antirecombinant human acid α-glucosidase (rhGAA) antibody titers, titer courses, and relation to genotype. (a) Geometric mean and 95% confidence intervals of anti-rhGAA antibody titer course during enzyme replacement therapy for the total group and separately for patients with high titers, intermediate titers, and no to low titers. The median treatment duration at the last ELISA measurement was 35 months (range, 24–41 months; IQR, 33–37 months). There was no significant difference between the three antibody titer groups with respect to treatment duration. Titers up to 1:250 were found to be technical background, which is indicated by the grey zone in the figure. (b) Distribution of the maximal anti-rhGAA antibody titers. Based on the highest antibody titer measured, patients were divided into the following groups: (i) no or low titer (0–<1:1,250); (ii) intermediate titer (1:1,250–<1:31,250); and (iii) high titer (≥1:31,250). (c) Pie charts showing the distribution of the four most frequent pathogenic GAA variants on the second allele in combination with c.-32-13T>G (IVS1) on the first allele. DNA analysis was missing for two patients in the no-to-low-titer group and for one patient in the intermediate-titer group. One patient without the IVS1 variant (placed in the high-titer group) is not displayed in the chart.
Effect of GAA variants on antibody formation
Pathogenic effects of GAA variants include effects on protein expression levels and effects on enzymatic activity while leaving protein expression levels unchanged. Several studies suggest that unchanged endogenous protein expression lowers the chance of forming antibodies to recombinant proteins.1,2,15,27,28,29 All but one Pompe patient carried the c.-32-13T>G (IVS1) GAA variant on one allele and another pathogenic GAA variant on the second allele. The IVS1 variant allows 10–15% expression of a wild-type GAA protein. We hypothesized that the total amount of GAA protein expression (from both alleles) may affect formation of antibodies to rhGAA. To test this, the nature of the second allele was considered. The four most frequent variants of the second allele were c.525delT (no protein expression), c.2481+102_2646+31del (delex18; expression of a truncated protein), c.1548G>A (protein expression), and c.925G>A (protein expression). Six patients (23%) in the no-to-low-titer group and five patients (18%) in the intermediate-titer group carried the delex18 variant, but none of the patients of the high-titer group did ( Figure 1c ). The other variants were found in all titer groups. These findings may indicate that the delex18 variant is associated with a reduced risk of developing high antibody titers, but larger patient cohorts should be tested to confirm this suggestion.
IARs
Thirteen patients (18%) showed clinically confirmed IARs (Supplementary Table S3 online), of whom 12 were female (P = 0.001). General malaise, chills, and hyperthermia were the most frequently observed symptoms. The occurrence of IARs was related to the titer: only 1/28 (4%) patients in the no-to-low-titer group experienced IARs, 5/29 (17%) in the intermediate-titer group experienced IARs, and 7/16 (44%) in the high-titer group experienced IARs (P = 0.001; Figure 2a ). Furthermore, the total number of IARs that a patient experienced during the study period increased with higher antibody titers (ρ = 0.46, P < 0.001; Figure 2b ). Most patients experienced their first IARs within the first year of treatment (median onset of IARs at 9 months after ERT; range, 6 weeks–35 months ERT). IARs usually started during or hours after completion of the infusion. In one case, mild IARs occurred 24–48 hours after the start of infusion. At the end of the study, the occurrence of IARs had resolved in 10/13 patients within 29 months (median, 3 months). One patient with high titers discontinued ERT. Two patients had ongoing IARS at the end of the study: the patient with an intermediate titer had experienced IARs for 3 months and the patient with a high titer had IARs for 26 months. Almost all IARs were mild to moderate and treatable by slowing the infusion rate during the first hours of the infusion and by administration of antihistamines and/or corticosteroids. In summary, occurrence and frequency of IARs correlated with antibody titers, but symptoms in general were transient and treatable.
Infusion-associated reactions and in vitro neutralizing effects. (a) Number of patients with and without infusion-associated reactions (IARs) in each anti-recombinant human acid α-glucosidase (rhGAA) antibody titer group. (b) Correlation between the number of IARs and the maximal anti-rhGAA antibody titer (Spearman ρ = 0.46, P < 0.001). (c) Neutralizing effects of anti-rhGAA antibodies. Intracellular acid α-alglucosidase activity following incubation of GAA-deficient fibroblasts with rhGAA plus patient serum. Data are expressed as percentage of control serum (fetal bovine serum). Tests were performed for all 16 patients with high peak titer and at multiple time points when titers ranged from 1:50 to 1:156,250 (•), for comparison of three patients with an intermediate titer (♦), and for two patients with no to low titer (▴).
Neutralizing effects of anti-rhGAA antibodies
Figure 2c shows the effect of patients’ sera on the uptake of GAA enzyme activity. In the high-titer group, 8/16 patients had extremely high maximum titers of ≥1:156,250. Patients’ sera with these very high titers were tested for neutralizing effects in vitro ( Figure 2c ). Individual neutralizing effects ranged from 15 to 85%. The analysis for the same patients was then extended with additional time points at which titers ranged from 1:50 to 1:156,250. The threshold titer at which neutralizing effects were observed was 1:31,250. This result prompted us to also analyze the eight remaining patients of the high-titer group with a maximum titer of 1:31,250. These patients showed neutralizing effects of 0–15%. As a final test, sera from three patients from the intermediate-titer group and two patients from the no-to-low-titer group were tested. None of these sera showed neutralizing effects. Altogether, the height of the titer and the neutralizing effect in cells correlated (ρ = −0.88, P < 0.001; Figure 2c ). In all but one patient, concomitant inhibition of enzyme activity in the medium was found (ρ = −0.59, P = 0.001; Supplementary Figure S2a online). The extent to which enzyme activity was inhibited in the medium correlated to that observed in cells (ρ = 0.67, P < 0.001; Supplementary Figure S2b online).
Effect of antibodies on clinical outcome
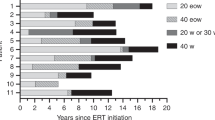
MRC sum score and FVC in the upright and in supine positions were determined for the three antibody titer groups. There were no significant differences between these groups at baseline ( Table 1 ) or after 3 years of ERT. In all three titer groups, patients with improving, stable, and declining clinical courses were present, suggesting that other factors besides antibody formation may also be involved in the response to ERT, as described previously.22,30,31 However, owing to small group sizes, statistical power is limited and effects of antibody formation on therapeutic efficacy could be overlooked. Therefore, the eight patients (11%) with very high maximal antibody titers (≥1:156,250) were further analyzed on an individual basis because they were considered to be at the highest risk for possible interference of antibodies with ERT ( Figure 3 ; % rhGAA activity in cells is indicated by a green asterisk; the green area marks the titer of 1:31,250, above which the neutralizing effects can be severe). Patient 1 showed clear evidence of interference with ERT by anti-rhGAA antibodies: ELISA titers were extremely high (1:3,906,250) and showed strong neutralizing effects, whereas MRC sum scores and FVC continued to decline. Patients 2–4 showed strong neutralizing effects of anti-rhGAA antibodies. However, it was unclear to what extent ERT had an effect on clinical outcome and whether antibodies interfered with ERT due to high stable MRC sum scores and FVC. In the remaining patients, anti-rhGAA antibodies either showed no neutralizing effect (patient 8) or neutralizing effects were only temporarily present and resolved over time (patients 5–7). Taking the results together, only 1/73 (1%) adult Pompe patients showed clear interference of anti-rhGAA antibodies with ERT.
Clinical outcome, antirecombinant human acid α-glucosidase (rhGAA) antibody titers, and neutralizing effects in eight patients with a very high maximal antibody titer. Each graph represents an individual patient. For each patient, the course of the Medical Research Council (MRC) sum score (squares, expressed in percentage of normal muscle strength) and forced vital capacity (FVC) in the upright (diamonds) and supine (triangles) positions (expressed in percentage of the predicted normal values) are shown before and after the start of enzyme replacement therapy (left Y-axis). Anti-rhGAA ELISA titers are shown (right Y-axis). The corresponding effect of the antibody on intracellular GAA activity (see Figure 2c) is indicated (%, left Y-axis). The green area indicates the titer range of 0 to 1:31,250, in which neutralizing effects of antibodies ranged from 0 to 50% and were considered moderate.
Discussion
This is the first study in which the long-term effects of anti-rhGAA antibodies on clinical outcome have been investigated in a large cohort of adult Pompe patients. More than half of the patients developed anti-rhGAA antibodies. High antibody titers were associated with increased number and frequency of IARs, which were manageable. The GAA genotype may be used to predict formation of high antibody titers. Only one patient (1% of total) showed a high antibody titer that probably interfered with ERT, suggesting that antibody formation is not a major threat to the efficacy of rhGAA treatment in the majority of adult Pompe patients.
Although previous reports have described IARs in patients receiving ERT, the relationship between high anti-rhGAA antibody titers and IARs found here is new. The most relevant IARs related to antibody titers were general malaise, chills, and hyperthermia. In most patients, the adverse reactions could be managed by slowing the infusion rate in the first hours of the infusion and by administration of antihistamines and/or corticosteroids. Patient 1 ( Figure 3 ) was the only patient in whom IARs could not be managed, which contributed to the cessation of ERT treatment at 33 months.20 These results suggest that the determination of antibody titers can help to predict which patients are at low risk for developing IARs, if confirmed in further studies. A remarkable finding was that all but one of the patients who developed IARs were female. We found that this was independent of age or menopausal status. There was no effect of gender on antibody titers or on the presence of neutralizing antibodies. This suggests that females may be more prone to developing IARs than males. It would be worthwhile to include assessment of IARs in future studies to investigate the extent of gender-specific development of IARs.
None of the patients with the IVS1/delex18 GAA genotype developed high titers, but this genotype occurred in 20% patients in the no-to-low- and intermediate-titer groups ( Figure 1c ). This may be explained as follows: both the IVS1 and the delex18 alleles allow residual expression of GAA protein, which may help to prevent an immune response against exogenous rhGAA. This is in contrast to genotypes such as IVS1/c.525delT, in which only the IVS1 allele produces GAA protein, whereas the c.525delT allele fails to express GAA protein.32,33 We hypothesize that the chance of anti-rhGAA antibody formation may depend on the protein expression levels of endogenous GAA. This is in agreement with the notion that negative cross-reactive immunologic material status in classic infantile Pompe patients is associated with a higher chance of antibody formation.1,2,15,27 Also, in X-linked Fabry disease, antibody formation can be prevented by the expression of endogenous α-galactosidase.34 Studies involving mouse models for Pompe disease showed prevention of antibody formation after genetic introduction of GAA variants that allowed protein expression.28,29 Taking these findings together, we provide evidence that the IVS1/delex18 GAA genotype may be a predicting factor for reduced risk of antibody formation.
On the group level, no effect of anti-rhGAA antibodies on pulmonary function or skeletal muscle strength was found. When the 11% of patients with the highest antibody titers were examined on an individual basis, only one patient showed clear evidence for interference of anti-rhGAA antibodies with ERT based on extremely high titers of neutralizing antibodies combined with profound clinical decline. Previous work has shown that in this patient, approximately half of the infused rhGAA is neutralized by circulating anti-rhGAA antibodies.20 For three patients (4%) with high anti-rhGAA antibody titers and neutralizing effects, the scores for pulmonary function and skeletal muscle strength were (close to) normal, and the effects of ERT and possible interference of antibodies were unclear and should be further monitored in the future. Most previous reports on antibody formation in adults are case reports, including a study involving three adults with high peak antibody titers and clinical decline.21 The Late Onset Treatment Study is the only available study with larger patient numbers, 59 adults during 18 months of follow-up, and it found no impeding effects of antibodies on treatment outcome.4 However, to date, formation of antibodies to rhGAA is still considered to indicate a potential risk for interference with treatment efficacy in adult Pompe patients, and large-scale studies with long follow-up are required to further address this issue. The results of the present study are positive news for adult Pompe patients treated with ERT.
The situation in adults contrasts sharply with that in infants with Pompe disease. Patients with the classic infantile phenotype have close to zero GAA enzymatic activity and are much more dependent on ERT compared with adult patients, who have some residual enzymatic activity. Without treatment, patients with the classic infantile phenotype die within 1 year, whereas untreated adult patients can develop serious symptoms at any point during their lives up to the age of 60 or beyond, with slow disease progression.16,17,18,19,35 Antibody titers are very high and neutralizing more often with the classic infantile phenotype, particularly in cross-reactive immunologic material–negative patients, because of the low presence or absence of endogenous GAA protein. Therefore, the higher frequency of patients who develop neutralizing antibodies combined with the higher dependency on rhGAA may explain why anti-rhGAA antibody formation poses a bigger threat to treatment outcome in infants with classic Pompe disease compared with adult patients. Nevertheless, the single adult patient in whom antibody formation clearly interferes with efficacy of ERT shows that adult patients should also be carefully monitored. Regular (every 6–12 months) screening of anti-rhGAA antibody formation using ELISA would be the first test that may be part of a standard clinical follow-up. When titers of 31,250 or higher are measured, a subsequent test may be performed to assess neutralizing effects of anti-rhGAA antibodies. Studies are ongoing in which immunomodulation is used to counteract anti-rhGAA antibody formation in patients with classic infantile Pompe disease .36,37,38,39 These studies may be valuable for possible interference with anti-rhGAA antibody formation in adult Pompe patients.
Author Contributions
J.d.V., E.K., Pv.D., A.T.v.d.P., and W.P. wrote the manuscript and designed the study. J.d.V., E.K., M.H., M.A.K., S.W., M.S., N.v.B., M.E.K., and W.P. analyzed and interpreted the data. J.d.V., E.K., M.H., M.A.K., S.W., and M.S. acquired the data. D.R. performed the statistical analyses. P.v.D., A.T.v.d.P., and W.P. supervised and coordinated the study.
Disclosure
A.T.v.d.P. has provided consulting services for various industries in the field of Pompe disease under an agreement between these industries and Erasmus MC, Rotterdam, the Netherlands. The other authors declare no conflict of interest.
References
Banugaria SG, Prater SN, Ng YK, et al. The impact of antibodies on clinical outcomes in diseases treated with therapeutic protein: lessons learned from infantile Pompe disease. Genet Med 2011;13:729–736.
Kishnani PS, Goldenberg PC, DeArmey SL, et al. Cross-reactive immunologic material status affects treatment outcomes in Pompe disease infants. Mol Genet Metab 2010;99:26–33.
Nicolino M, Byrne B, Wraith JE, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med 2009;11:210–219.
van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med 2010;362:1396–1406.
Brands MM, Hoogeveen-Westerveld M, Kroos MA, et al. Mucopolysaccharidosis type VI phenotypes-genotypes and antibody response to galsulfase. Orphanet J Rare Dis 2013;8:51.
Clarke LA, Wraith JE, Beck M, et al. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics 2009;123:229–240.
Harmatz P, Giugliani R, Schwartz I, et al. Long term benefit and safety with recombinant human arylsulfatase, B (rhASB) ERT for MPS VI [abstr]. J Inherit Metab Dis 2006;29(suppl 1):29.
Kakkis ED, Muenzer J, Tiller GE, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. New Engl J Med 2001;344:182–188.
Muenzer J, Wraith JE, Beck M, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome). Genet Med 2006;8:465–473.
Hollak CE, Linthorst GE. Immune response to enzyme replacement therapy in Fabry disease: impact on clinical outcome? Mol Genet Metab 2009;96:1–3.
Vedder AC, Breunig F, Donker-Koopman WE, et al. Treatment of Fabry disease with different dosing regimens of agalsidase: effects on antibody formation and GL-3. Mol Genet Metab 2008;94:319–325.
Wilcox WR, Linthorst GE, Germain DP, et al. Anti-α-galactosidase A antibody response to agalsidase beta treatment: data from the Fabry Registry. Mol Genet Metab 2012;105:443–449.
Hirschhorn R, Reuser AJJ. Glycogen storage disease type II; acid alpha-glucosidase (acid maltase) deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill: New York, 2001:3389–3420.
van der Ploeg AT, Reuser AJJ. Lysosomal storage disease 2 - Pompe’s disease. Lancet 2008;372:1342–1353.
van Gelder CM, Hoogeveen-Westerveld M, Kroos MA, Plug I, van der Ploeg AT, Reuser AJ. Enzyme therapy and immune response in relation to CRIM status: the Dutch experience in classic infantile Pompe disease. J Inherit Metab Dis 2015;38:305–314.
Hagemans ML, Hop WJ, Van Doorn PA, Reuser AJ, Van der Ploeg AT. Course of disability and respiratory function in untreated late-onset Pompe disease. Neurology 2006;66:581–583.
Hagemans ML, Winkel LP, Van Doorn PA, et al. Clinical manifestation and natural course of late-onset Pompe’s disease in 54 Dutch patients. Brain 2005;128(Pt 3):671–677.
Müller-Felber W, Horvath R, Gempel K, et al. Late onset Pompe disease: clinical and neurophysiological spectrum of 38 patients including long-term follow-up in 18 patients. Neuromuscul Disord 2007;17:698–706.
van der Beek NA, de Vries JM, Hagemans ML, et al. Clinical features and predictors for disease natural progression in adults with Pompe disease: a nationwide prospective observational study. Orphanet J Rare Dis 2012;7:88.
de Vries JM, van der Beek NA, Kroos MA, et al. High antibody titer in an adult with Pompe disease affects treatment with alglucosidase alfa. Mol Genet Metab 2010;101:338–345.
Patel TT, Banugaria SG, Case LE, Wenninger S, Schoser B, Kishnani PS. The impact of antibodies in late-onset Pompe disease: a case series and literature review. Mol Genet Metab 2012;106:301–309.
de Vries JM, van der Beek NA, Hop WC, et al. Effect of enzyme therapy and prognostic factors in 69 adults with Pompe disease: an open-label single-center study. Orphanet J Rare Dis 2012;7:73.
van der Ploeg AT, Barohn R, Carlson L, et al. Open-label extension study following the Late-Onset Treatment Study (LOTS) of alglucosidase alfa. Mol Genet Metab 2012;107:456–461.
Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5–40.
Wilson SH, Cooke NT, Edwards RH, Spiro SG. Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax 1984;39:535–538.
Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York: Springer, 2000.
Abbott MA, Prater SN, Banugaria SG, et al. Atypical immunologic response in a patient with CRIM-negative Pompe disease. Mol Genet Metab 2011;104:583–586.
Raben N, Nagaraju K, Lee A, et al. Induction of tolerance to a recombinant human enzyme, acid alpha-glucosidase, in enzyme deficient knockout mice. Transgenic Res 2003;12:171–178.
Nayak S, Doerfler PA, Porvasnik SL, et al. Immune responses and hypercoagulation in ERT for Pompe disease are mutation and rhGAA dose dependent. PLoS One 2014;9:e98336.
Bembi B, Pisa FE, Confalonieri M, et al. Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. J Inherit Metab Dis 2010;33:727–735.
Regnery C, Kornblum C, Hanisch F, et al. 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy. J Inherit Metab Dis 2012;35:837–845.
Kroos MA, Pomponio RJ, Hagemans ML, et al. Broad spectrum of Pompe disease in patients with the same c.-32-13T->G haplotype. Neurology 2007;68:110–115.
Huie ML, Chen AS, Brooks SS, Grix A, Hirschhorn R. A de novo 13 nt deletion, a newly identified C647W missense mutation and a deletion of exon 18 in infantile onset glycogen storage disease type II (GSDII). Hum Mol Genet 1994;3:1081–1087.
Wilcox W, Gruskin D, Warnock D. Few females develop anti-alpha-galactosidase A IgG antibodies in response to agalsidase beta treatment: data from the Fabry Registry. Mol Genet Metab 2011;102:S46–S47.
van den Hout HM, Hop W, van Diggelen OP, et al. The natural course of infantile Pompe’s disease: 20 original cases compared with 133 cases from the literature. Pediatrics 2003;112:332–340.
Mendelsohn NJ, Messinger YH, Rosenberg AS, Kishnani PS. Elimination of antibodies to recombinant enzyme in Pompe’s disease. N Engl J Med 2009;360:194–195.
Banugaria SG, Prater SN, McGann JK, et al. Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from Pompe disease. Genet Med 2013;15:123–131.
Elder ME, Nayak S, Collins SW, et al. B-Cell depletion and immunomodulation before initiation of enzyme replacement therapy blocks the immune response to acid alpha-glucosidase in infantile-onset Pompe disease. J Pediatr 2013;163:847–54.e1.
Lacaná E, Yao LP, Pariser AR, Rosenberg AS. The role of immune tolerance induction in restoration of the efficacy of ERT in Pompe disease. Am J Med Genet C Semin Med Genet 2012;160C:30–39.
Acknowledgements
The authors thank all the patients for their participation in the study. The authors also acknowledge Arnold Reuser of the Department of Clinical Genetics, Erasmus MC University Medical Center, Rotterdam, the Netherlands, for his advice and discussion. The advice by B.C. Jacobs of the Departments of Neurology and Immunology, Erasmus MC University Medical Center, on the design of the study is greatly appreciated.
The work on Pompe disease at Erasmus MC is supported by the Prinses Beatrix Spierfonds/Stichting Spieren voor Spieren (projects OP07-08 and W.OR 13–21), Sophia Children’s Hospital Foundation (grant S-687), and ZonMW, the Netherlands Organization for Health Research and Development (project 152001005).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures and Tables
(ZIP 617 kb)
Rights and permissions
About this article
Cite this article
de Vries, J., Kuperus, E., Hoogeveen-Westerveld, M. et al. Pompe disease in adulthood: effects of antibody formation on enzyme replacement therapy. Genet Med 19, 90–97 (2017). https://doi.org/10.1038/gim.2016.70
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2016.70
Keywords
This article is cited by
-
Home-based enzyme replacement therapy in children and adults with Pompe disease; a prospective study
Orphanet Journal of Rare Diseases (2023)
-
Home-Based Infusion of Alglucosidase Alfa Can Safely be Implemented in Adults with Late-Onset Pompe Disease: Lessons Learned from 18,380 Infusions
BioDrugs (2023)
-
Survey on the management of Pompe disease in routine clinical practice in Spain
Orphanet Journal of Rare Diseases (2022)
-
Antibodies against recombinant human alpha-glucosidase do not seem to affect clinical outcome in childhood onset Pompe disease
Orphanet Journal of Rare Diseases (2022)
-
Three-dimensional tissue-engineered human skeletal muscle model of Pompe disease
Communications Biology (2021)