Abstract
Purpose: To describe the clinical and public health activities of all entities involved in long-term follow-up of children with conditions identified by newborn dried bloodspot screening, including the requirements for interoperable clinical and public health information systems that will support care from birth through adulthood.
Methods: The Southeastern Newborn Screening Genetics Collaborative (Region 3) convened a workgroup of experts in pediatric care, genetics, and public health, facilitated by the Public Health Informatics Institute (the Institute). The Institute's Collaborative Requirements Development Methodology was used.
Results: Three overlapping steps in the long-term follow-up process were examined: needs assessment, ongoing treatment/management, and knowledge generation. In addition, greater definition was given to the roles of Clinical Care Coordinator and Public Health Care Coordinator, as defined by a previous workgroup, and a new role was identified—Care Plan Leader (primary care provider or specialist) who would serve as treatment coordinator and centralize long-term follow-up care provision to minimize gaps in clinical care.
Conclusion: The outcome of the Region 3 Workgroup's efforts is an expanded notion of long-term follow-up to extend throughout the patient's lifespan, with an emphasis on the coordination of care involving both clinical and public health sectors and on requirements for interoperable clinical and public health information systems.
Similar content being viewed by others
Main
The clinical services for children with a confirmed genetic or metabolic disorder following newborn dried bloodspot screening (NDBS) includes primary care and condition-specific specialty care, carried out through the lifetime of the individual (i.e., long-term follow-up [LTFU]). In recognition of the ongoing services required for the intervention and management of children with special health care needs, the Department of Health and Human Services Secretary's Advisory Committee on Heritable Disorders in Newborns and Children described the LTFU process as inclusive of the following elements: care coordination through a medical home, evidence-based treatment, continuous quality improvement, and new knowledge discovery.1 The American Academy of Pediatrics defines the medical home as family-centered care that follows children from birth through adolescence2; the Association of Maternal and Child Health Programs defines LTFU as proceeding from the initiation of treatment through 21 years of age.3 However, there has not been much involvement in LTFU on the part of public health agencies. As Hoff4 observes, “Many state NBS programs have well defined STFU [short-term follow-up] protocols that accompany their testing activities. However, long-term follow-up (LTFU) is a more controversial, less understood component of the NBS system in the United States.” His study of 38 state newborn screening programs discovered specific practices that may result in the limited nature of LTFU from a public health perspective including the preemptive emphasis on short-term follow-up “… that obscures the longer-term follow-up focus …” This occurs, Hoff notes, despite the availability in some states of staff and resources as well as the recognition that, as testing panels expand, longitudinal data must be gathered and long-term treatment outcomes assessed. The result is “… the absence of standardized criteria for organizing and conducting LTFU nationally …”4
In response to the need for a more inclusive and comprehensive approach to LTFU for children identified through NDBS screening, the Southeast Newborn Screening and Genetics Collaborative (SERC/Region 3) convened a workgroup of experts in pediatric care, genetics, and public health to describe the clinical and public health roles and responsibilities of all entities involved in LTFU (SERC is one of seven regional collaboratives funded by the Health Resources and Services Administration to strengthen and support the genetics and newborn screening capacity of the states). As facilitated by the Public Health Informatics Institute (the Institute), the scope of this work also included detailing the requirements involved in building the specifications for interoperable clinical and public health information systems that will support care from birth through adulthood. The ability of these systems to communicate is essential to the effective implementation of any LTFU plan. As Hinman and Davidson5 observed, “Existing health information systems have demonstrated that they can improve health services, although most are not able to share information. Additional benefits are bound to accrue when, for example, a pediatrician seeing a child for a well-infant visit is alerted by the information system that the child failed a newborn hearing screening test but has not yet been fully evaluated, diagnosed, or treated. Because so few information systems are currently integrated, it is not yet possible to document these benefits.”
The ability of clinical and public health information systems to “communicate” through the exchange of information is essential to the effective implementation of any LTFU plan that includes all entities involved in the screening process and in the clinical care and management of patients. Moreover, improvements in science and technology have generated highly sensitive testing mechanisms, making it possible to screen for an expanded number of conditions. Therefore, the need to track these patients and share the long-term outcomes from a population health standpoint becomes more important. However, there are currently no regional or national information systems through which a clinical or public health practitioner or other stakeholder can coordinate all the medical, social, and ancillary needs of their patients identified through NDBS. Hence, an objective of the Region 3 Workgroup was to clearly describe and detail the roles and responsibilities of all entities involved in newborn screening and care. Defining the core roles and activities of all entities within the scope of LTFU is the initial step in defining requirements for intrastate and interstate health information systems. These information exchange systems can support the informational needs of all the stakeholders involved in the overall NDBS system. Such an analysis also contributes to the development of future information systems that will conform to National Health Information Network interoperability standards.
In addition, any national LTFU should be employed via strategic health information exchanges (HIEs)—a term that has now become commonplace to describe the collaborative network of stakeholders convened to share health information, resolve health care costs, and reduce medical errors through electronic transport of health information. The benefits of an HIE in relation to LTFU in newborn screening is a centralized means of facilitating the flow of information among all entities involved in long-term care including clinicians, public health entities, families, and ancillary services—thereby establishing a centralized means of care coordination. A key value of an HIE is the improvement of individual health outcomes by providing access to more complete information. The use of HIEs would also allow the information regarding long-term care treatment and outcomes to be evaluated from a population health perspective, potentially ensuring the improvement of community as well as individual health and improving the availability of evidenced-based medicine.
This article describes the rethinking and requirements development steps (terms defined by the Institute's Requirement Development Methodology), and expands on the roles and activities of the Clinical Care Coordinator (CCC) and Public Health Care Coordinator (PHCC) in LTFU, as identified by a previous workgroup (the NDBS Workgroup convened by the Institute and Region 3 under the aegis of the Maternal and Child Health Bureau, Health Resources and Services Administration),6 and identifies the role of Care Plan Leader (primary care provider [PCP] or specialist). Defining these core activities within the scope of LTFU is the initial step in defining requirements for intrastate and interstate information systems. These information exchange systems can support the informational needs of all the stakeholders involved in the overall NDBS system. Such an analysis also contributes to the development of future information systems that will conform to National Health Information Network interoperability standards.
MATERIALS AND METHODS
The SERC (Region 3) convened a workgroup of experts involved in various aspects of newborn screening and LTFU including clinicians, health department representatives, and a parent of a child with a condition identified through NDBS. The Region 3 Workgroup was facilitated by the Institute and used the Institute's collaborative Requirements Development Methodology to review the business process analysis (BPA) previously carried out, rethink the processes and modify the context diagrams and task flows, and develop the interoperable clinical and public health information system requirements. The tools used in the methodology included the following:
-
Business process matrix—identifying the goals, objectives, business rules, triggers, task sets, inputs, outputs, and measurable outcomes.
-
Context diagrams—illustrating the participants and flow of information within the work environment. Context diagrams consist of two graphical elements: circles and arrows. The circles represent entities (a person or group of persons who perform one or more tasks involved in the process depicted). Arrows represent transactions that involve the exchange of information among entities.
-
Task flow charts—capturing the basic temporal flow of tasks and the individual or groups responsible for each task. Graphical elements in a task flow diagram depict inputs, processes, and results for each step that make up a task. Each of the individuals or groups has its own horizontal area (swim lane). The graphical elements (inputs, processes, and results) may remain in one swim lane, indicating that the task is confined to that person or group, or may cross two or more swim lanes, in which case the task that these elements depict can be carried out by any of the entities displayed in those swim lanes.7
In a previous publication, the authors described the clinical and public health components and activities of the NDBS process as identified by a workgroup of experts.6 The collaborative process involved carrying out a BPA of the activities as a first step toward identifying the requirements for information systems to support the NDBS process for a child identified through screening from the birth through LTFU. The Institute defines BPA as, “… the effort to understand an organization and its purpose while identifying the activities, participants, and information flows that enable the organization to do its work. The output of the business process analysis phase is a model of the business processes consisting of a set of diagrams and textual descriptions to be used for design or redesign of business processes.”7
Once the BPA is completed, the next steps are to rethink the business processes to determine whether they could be improved and then to develop the requirements for the information systems. The number of people/agencies involved in the NDBS process makes it critical that information systems have the capacity and capability of providing needed information to anyone involved in the process. Identifying the requirements for information systems that support these activities begins with an analysis of who does what with whom—the business process.6 This task was achieved by the previous workgroup. The charge of the second Region 3 Workgroup was in the redesign of these business processes to refine the roles and responsibilities of all entities involved in the care of children with special needs and to expand the notion of LTFU to include both clinical and public health sectors. The Institute defines business process redesign as, “The effort to improve the performance of an agency's business processes … and seeks to restructure tasks and workflow to be more effective and more efficient.”7
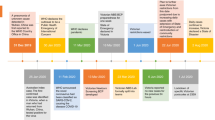
The Region 3 Workgroup convened two facilitated face-to-face meetings (January 30–31 and May 1–2, 2009) and telephonic/electronic discussions to advance the effort and revise documents as they were developed. The Workgroup concentrated on care coordination/ongoing treatment, with some attention to knowledge generation. Subsequent activities will complete development of requirements for knowledge generation in greater detail as well as the development of requirements for knowledge management and dissemination, and continuous quality improvement (Fig. 1).
RESULTS
The outcome of the Region 3 Workgroup's efforts is an expanded notion of LTFU as following throughout his or her lifespan a child who screened positive through NDBS and was subsequently confirmed with a genetic or metabolic disorder and to include both clinical care and public health care coordination in the management of care and services. The former would be an integral member of the medical home and the latter an extension of the public health role in assuring continuity of care. Both of these coordinators would be involved in the assurance of care (provision and implementation) and would facilitate communication among all members of the medical home. The CCC ensures the presence of a core clinical team at each step to prevent the possibility of the patient becoming one of the many patients who are lost to follow-up. The role of CCC might be fulfilled and/or shared by a nurse practitioner, dietitian, or other clinical specialists. Among the CCC's duties would be to ensure that no gaps in care occur by adjusting the care plan to meet patient health care needs and to inform the family as necessary. In addition, the CCC would coordinate services with other clinical experts, such as the gastroenterologist for feeding tube placement or radiologist for liver scans. Other CCC functions may include generating emergency care updates for providers and families, notifying schools of the need for special meals and medications, and ensuring that providers follow-up with immunizations. The PHCC would interact with the larger public health realm (local, state, and national) to deliver/transmit outcome and service data necessary for population health provision; public health and CCC, both entities in turn would contribute data to the research community as part of its knowledge generation function. It should be noted that care coordination differs from case management, which involves facilitation and advocacy of services to meet an individual's health care needs conducted by a nurse or social worker.
The expanded notion of LTFU as outlined by the Region 3 Workgroup reflects the necessary response of the clinical and public health teams to the shifting care needs of the patient depending on condition and age. Ongoing dialog would be necessary among all members involved in care to redefine roles and responsibilities to best meet individual patient needs from infancy to adolescence and through adulthood. The fluid nature of the medical home would make necessary the concept of comanagement of care between the PCP and specialist, with one taking on the role of Care Plan Leader. The Care Plan Leader centralizes LTFU care provision to minimize gaps in medical care. The role would be determined by patient need and would be condition-dependent so that both generalist and specialist services and decision support would be rendered in a collaborative manner, while one or the other serves as a lead decision-maker.
In reviewing and rethinking the LTFU business processes, the Region 3 Workgroup members felt it useful to separate care coordination/ongoing treatment into two individual (but related) business processes—needs assessment and ongoing treatment. The context diagram and task flowchart for these processes as described by the previous NDBS Workgroup were revised accordingly. Figure, Supplemental Digital Content 1, http://links.lww.com/GIM/A139 shows the revised context diagram for ongoing treatment, and Figures, Supplemental Digital Content 2 and 3, http://links.lww.com/GIM/A140 and http://links.lww.com/GIM/A141 show the revised task flowchart for ongoing treatment. Each activity in the flowchart is identified by a number. As shown in Figures, Supplemental Digital Content 2 and 3, http://links.lww.com/GIM/A140 and htp://links.lww.com/GIM/A141 for the clinical team the Region 3 Workgroup described separately the activities of the PCP, specialist, CCC, and the new role of Care Plan Leader.
During the redesign exercise, the Region 3 Workgroup also added another entity, the Knowledge Generation Group. The latter would be an entity external to the core medical home group and involved in the reporting/notification, surveillance, and clinical trial information exchange among clinical and public health entities. This entity is reflected in the addition of a swim lane to the task flow diagram.
The Region 3 Workgroup subsequently developed information system requirements for each of the activities. Table 1 shows the activities in which the CCC is involved and the information system requirements for those activities. Table 2 shows the activities in which the PHCC is involved and the information system requirements for those activities. A more complete report showing the business process matrix, context diagrams, flow charts, and information system requirements for the two business processes is available at http://southeastgenetics.org/(Requirements for Newborn Dried Bloodspot Screening Long-term Follow-up Information Systems. Linking Clinical and Public Health Information Systems will be posted simultaneously with publication of this article).
DISCUSSION
This work extends and expands on the work of the previous workgroup in further defining the roles of the CCC and the PHCC, identifying a new role of Care Plan Leader, and identifying the information system requirements for all entities involved in LTFU. One thing that must be emphasized is that the information system is not merely a repository of information. It is also a provider of information and should offer clinical decision support by providing condition-specific information to the provider, including treatment/management guidelines, availability of community resources, and so on. It must be capable of providing information to, and receiving information from, the other medical, social, and ancillary treatment services. It must also be capable of providing information to other information systems involved in knowledge generation.
An important set of activities in the Needs Assessment process is obtaining and documenting the family/patient's consent to have information submitted to the Knowledge Generation Group. Consent for sharing information for knowledge generation will be required, although consent may not be required for public health surveillance purposes. Information system requirements related to consent also demonstrate interactive functions. Requirements include the following:
-
Must capture consent or nonconsent that a patient is allowing data to be shared with Knowledge Generation Group. Must also capture types of consent.
-
Must generate a patient consent form for the patient to sign for submitting patient-related data to the Knowledge Generation Group.
-
Must prompt the Care Plan Leader and CCC when reconsent is needed by the patient for submitting patient-related data to the Knowledge Generation Group.
-
Must define which roles will have access to which patient data. This will be based on the level of consent provided by the patient.
-
Must prompt the CCC to gain consent for patient data sharing when the needs assessment is performed.
Another important activity in the needs assessment process is identification of a Care Plan Leader. The PCP and the specialist will jointly decide who is to be the Care Plan Leader for condition-specific care. The decision will be based on factors including the condition, geography/access, insurance coverage, family preference, and so on. In any event, the specialist and the PCP will remain in communication with one another.
As stated in the earlier article, many of the activities described for LTFU represent “aspirational practices” because not many states have well-coordinated functioning LTFU programs. To date, feedback on the activities described in the earlier article has been positive,8 and the authors believe there will be general agreement with the extension/expansion described here.
After the next steps are completed—completing development of information requirements for knowledge generation and developing requirements for knowledge dissemination and continuous quality improvement—it should be possible for NDBS LTFU programs to approach information system vendors with the requirements and request development of information systems to meet those needs. It will also be possible for individual programs to modify requirements to meet their particular needs. However, ease of information sharing would be much greater if programs adopted the same basic framework. The authors hope the activities and requirements defined here will serve the needs of programs around the country (and perhaps beyond) and that information systems will be developed to serve all the needs for NDBS LTFU—care coordination through a medical home, evidence-based treatment, continuous quality improvement, and new knowledge discovery.
GLOSSARY OF TERMS
Confirmatory/diagnostic testing (Clinical and Laboratory Standards Institute)
Test to prove or disprove the presence of a specific condition identified by screening tests (for NDBS screening, this testing is from a specimen other than the screening specimen).
Context diagram
A nontechnical graphical tool for recording context information. It consists of the following elements: (1) entity—a person or group of people (e.g., accounts payable clerk or accounts payable department) who performs one or more tasks involved in a process; and (2) transaction—information exchanges between entities. Entities are represented by circles and transactions are represented by arrows. A context diagram may involve all the transactions of a single user of a system or of multiple users. Usually, single-user diagrams are attempted first (for ease), but multiuser diagrams are needed to get a good look at an entire process.
Care plan leader
The Care Plan Leader centralizes LTFU care provision to minimize gaps in medical care. The role would be determined by patient need and would be condition-dependent so that both generalist and specialist services and decision support would be rendered in a collaborative manner, while one or the other serves as a lead decision-maker.
Clinical care coordinator
As a member of the LTFU team, the CCC is responsible for ensuring that the patient receives the range of appropriate services from the point of diagnosis through adulthood.
Entity
A person, group, organization, or system that interacts through transactions. Entities are the participants in a process and are represented by circles in the context diagrams.
Goal
The major health goal that the business process supports. The goal is the end state to be achieved by the work of the health agency and should be defined in terms of the benefits provided to the community/population or individual/client.
Input
Information received by the business process from external sources. Inputs are not generated within the process.
Objective
A concrete statement describing what the business process seeks to achieve. The objective should be specific to the process such that one can evaluate the process or reengineer the process and understand how the process is performing toward achieving the specific objective. A well-worded objective will be SMART (Specific, Measurable, Attainable/Achievable, Realistic, and Time-bound).
Outcome
The resulting transaction of a business process that indicates the objective has been met. Producing or delivering the outcome satisfies the stakeholder of the first event that triggered the business process. Often, measures can be associated with the outcome (e.g., how much, how often, decrease in incidents, and so on). Please note that an outcome can be, but is not necessarily, an output of the process.
Output
Information transferred out from a process. The information may have been the resulting transformation of an input, or it may have been information created within the business process.
Public health care coordinator
As a member of the LTFU team, the PHCC assesses the completeness of care and provides assurance of the delivery of care.
Stakeholder
A person, group, or business unit that has a share or an interest in a particular activity or set of activities.
Task
A definable piece of “work” that can be done at one time. A business process is made up of a series of work tasks.
Task flow diagram
A graphical tool used to capture the basic flow of tasks ands the exception flow(s) identified through decision points. The graphical description of tasks shows inputs, processes, and results for each step that makes up a task.
Transaction
An information exchange among entities. Transactions are represented by arrows in context diagrams.
Trigger
Event, action, or state that initiates the first course of action in a business process. A trigger may also be an input, but not necessarily so.
REFERENCES
Kemper AR, Boyle CA, Aceves J, et al. Long-term follow-up after diagnosis resulting from newborn screening: statement of the US Secretary of Health and Human Services' Advisory Committee on Heritable Disorders and Genetic Diseases in Newborns and Children. Genet Med 2008; 10: 259–261.
The Medical Home. Medical Home Initiatives for Children with Special Needs Project Advisory Committee. American Academy of Pediatrics. Pediatrics 2002; 110: 184–186.
Association of Maternal and Child Health Programs. Newborn screening long-term follow-up assessment. Washington, DC: Association of Maternal and Child Health Programs, 2007. Available at: www.amchp.org. Accessed June 16, 2009.
Hoff T . Long-term follow-up culture in state newborn screening programs. Genet Med 2008; 10: 396–403.
Hinman AR, Davidson AJ . Linking children's health information systems: clinical care, public health, emergency medical systems, and schools. Pediatrics 2009; 123: S67–S73.
Hinman AR, Mann MY, Singh RH ; NDBS Business Process Analysis Workgroup. Newborn dried bloodspot screening: mapping the clinical and public health components and activities. Genet Med 2009; 11: 418–424.
Public Health Informatics Institute. Taking care of business: a collaboration to define local health department business processes. Available at: http://www.phii.org/resources/doc_topics.asp?id=20. Accessed May 21, 2009.
McCabe LL, McCabe ERB . Newborn screening as a system from birth through lifelong care. Genet Med 2009; 11: 409–410.
Acknowledgements
This work was supported by a grant from the Maternal and Child Health Bureau, Health Resources and Services Administration (HRSA/MCHB), Department of Health and Human Services (Title V, Social Security Act, Grant #2-U22 MC010979).
Sincere thanks are extended to the members of the Region 3 Workgroup, and the staffs of the Southeast Regional Newborn Screening and Genetics Collaborative and the Public Health Informatics Institute.
Southeastern Newborn Screening and Genetics Collaborative (Region 3) Workgroup: Sara Copeland, MD; Tina Cowan, PhD; Jim Eckman, MD; Lisa Feuchtbaum, DrPH, MPH; Alan Hinman, MD, MPH; Alex Kemper, MD, MPH, MS; David Kronn, MD; Jennifer Lail, MD; Brendan C. Lanpher, MD; Harvey Levy, MD; Nicola Longo, MD, PhD; John Parks, MD; Rani H. Singh, PhD, RD; K. C. Singletary, DVM; Phyllis Sloyer, PhD; Janet Thomas, MD; and Kathy Tomashitis, MNS, RD, LD.
Workgroup Members: Sara Copeland, MD, University of Iowa Genetics, Iowa City, Iowa; Jim Eckman, MD, Director, Hematology/Oncology, Emory University School of Medicine, Atlanta Georgia; Lisa Feuchtbaum, DrPH, MPH, Research Scientist Genetic Disease Branch, California Department of Health Services, Richmond, California; Alan R. Hinman, MD, MPH, Senior Public Health Scientist, Public Health Informatics Institute, Decatur, Georgia; Alex Kemper, MD, MPH, MS, Duke University Medical Center, Durham, North Carolina; David Kronn, MD, Director Inherited Metabolic Disease Center, Maria Fareri Children's Hospital at Westchester Medical Center, Hawthorne, New York; Jennifer Lail, MD, Chapel Hill Pediatrics, Chapel Hill, North Carolina; Brendan C. Lanpher, Division of Medical Genetics, Vanderbilt University School of Medicine, Nashville, Tennessee; Harvey Levy, MD, Division of Genetics, Children's Hospital, Boston, Massachusetts; Nicola Longo, MD, PhD, Departments of Pediatrics and Medical Genetics, University of Utah, Salt Lake City, Utah; John Parks, MD, Division of Pediatric Endocrinology, Emory University School of Medicine, Atlanta, Georgia; Rani H. Singh, Associate Professor, Department of Human Genetics, Emory University School of Medicine, Atlanta, Georgia; K. C. Singletary, DVM, Lafayette, Louisiana; Phillis Sloyer, PhD, Director, Children's Medical Services, Florida Department of Health, Tallahassee, Florida; Janet Thomas, MD, Director, Inherited Metabolic Diseases Clinic, University of Colorado, Aurora, Colorado; and Kathy Tomashitis, MNS, RD, LD, Pediatric Screening Follow-up Program, Department of Health and Environmental Control, Columbia, South Carolina.
Southeast Newborn Screening and Genetics Collaborative Staff: Courtney Nicole Coleman, MPH, Project Manager; Elizabeth Otwell, MSPH, Research Project Coordinator; and Adrya Stembridge, Applications Developer/Analyst.
Public Health Informatics Institute Staff: Matt Hawkins, MBA, Business Analyst; Jim Mootrey, Project Manager; and Srila Sen, MA, Communications Manager.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.geneticsinmedicine.org).
Rights and permissions
About this article
Cite this article
Singh, R., Hinman, A. Newborn dried bloodspot screening: Long-term follow-up activities and information system requirements. Genet Med 12 (Suppl 12), S261–S266 (2010). https://doi.org/10.1097/GIM.0b013e3181fe5f6c
Issue Date:
DOI: https://doi.org/10.1097/GIM.0b013e3181fe5f6c