Abstract
The effect of monoethylphosphate (MEP, commercial available or synthesized) together with IL-2 on the selective proliferation of human γδ T cells in vitro from peripheral blood mononuclear cells (PBMC) of healthy donors and of cancer patients was investigated. The γδ T cells were stimulated by MEP to proliferate in a dose-dependent manner. The effect of synthesized MEP was 10 times greater than that of commercial MEP. When the PBMCs of healthy donors were cultured for 25 d in the medium containing different concentrations of MEP, the total cell number increased about 1000-3000 fold; and the ratio of γδ T cells reached to 70-80%. The selective expansion of γδ T cells depended on the synergic action of MEP and IL-2. The bulk cultured γδ T cells exhibited obvious cytotoxic activities against allogenic tumor cell lines (SQ-5, K562 and Daudi) and autologous tumor cells. The culture system described here not only offers a simple method for obtaining a large number of γδ T cells which may become a new effector in the adoptive immunotherapy, but also provides a useful model for the further studies of the structure and function of γδ T cells in vitro.
Similar content being viewed by others
Introduction
Although many papers have reported the diversity, development and subtype heterogeneity of γδ T cells, their physiological role in the immune responses remains an important and unresolved question[i-3]. However, the accumulated experimental results provided the evidences for the specific functions of γδ T cells in immunity against bacterial pathogen and viral infection4, 5, 6, 7, 8, and in the anti-tumor activity in human9. Furthermore, the current studies suggest that γδ T cells recognition may be fundamentally different from that of αβ T cells10, 11. More importantly, the charateristics of the antigens recognized by γδ T cells are more clear now12, 13, 14, 15. The nonpeptide ligands for human peripheral blood γδ T cells were reported recently5, 16.
γδT cells comprise about 5% of the CD3+ T cells in human peripheral blood and lymphoid tissues17. Many attempts to expand these cells from the blood of healthy donors failed, because the cultures were quickly overgrown with αβ T cells1.
In our present study, we confirmed the effect of MEP (monoethylphosphate, commercial or synthesized, which was reported as one of the nonpeptide ligands for human γδ T cells) on the selective proliferation of γδ T cells from peripheral blood mononuclear cells (PBMC) of healthy donors and cancer patients. The establishment of a culture system for the stable and optimal growth of γδ T cells and the cytotoxicity of the cultured γδ T cells against some autologous and allogenic tumor cells will be presented.
Materials and Methods
Cell line and culture medium
The LAK-sensitive cell line Daudi, the NK-sensitive cell line K562 and the human lung squamous carcinoma cell line SQ-5 were provided by RIKEN Cell Bank. Recombinant human IL-2 was kindly provided by Dr. T. Hirakawa (Ajinomoto Co., Tokyo, Japan) or purchased from Shanghai Hua Xing High Bioteehnology Co., China. SQ-5, Daudi and K562 cells were maintained in MEM, RPMI-1640 and Ham's F12 supplemented with 10% FBS respectively at 37°C in a humidified 5% CO2 incubator. Medium RHAMa(+) was developed for the induction of human LAK cells in RIKEN Cell Bank18. It is consisted of a mixture of RPMI-1640, Ham's F-12, and MEMa at 3:1:1 by volume, supplemented with 0.02 mg/L a-tocopherol, 0.002 mg/L sodium selenite, 0.004 mg/L linoleie acid, 0.2 mg/ml cholesterol, 500 mg/ml human albumin, 0.6 mg/ml mercaptoethanol, 0.6 mg/ml ethanolamine, 5 mg/ml insulin, 5 mg/ml transferrin, and 66 mg/ml pyruvic acid.
Monoethyl phosphate (MEP)
The commercial MEP was purchased from Tokyo Kasei Co., Japan. It contained 42.5% pure monoethylphosphate. The synthesized MEP was produced and identified by the lab of Prof. Masataka Mochizuki (Kyoritsu College of pharmacy, Shibakoen, Minato-ku, Tokyo, Japan). The MEP was added according to the calculation of the pure MEP present in the commercial or synthesized preparations into mole solution.
Preparation and culture of Mononuclear cells
Heparinized venous peripheral blood samples were obtained from healthy human donors and cancer patients. Mononuclear cells (PBMC) were separated by conventional Ficoll-Hypaque density gradient centrifugation. PBMC were cultured at a concentration of 5×105 cells/ml in 24-well plates (Becton Dickinson). The culture medium was RHAMa(+) containing 5% autoplasma, IL-2 and MEP at indicated concentrations. The cells were cultured at 37°C in a humidified 5% CO2 incubator. Half of the medium was replaced with fresh medium on d 3. Subcultures were carried out every 2 d after 6 d of culture.
Flow cytometric analysis of γδ and αβ T cells
At indicated times, two colour direct immunofluorescence staining was performed on PBMC (freshly isolated or cultured) with phycoerythrin-conjugated anti-TCRα/δ-1 MAbs (Becton Dickinson) and fluorescein isothiocyanate-conjugated anti-TCRα/β-1 MAbs (Becton Dickinson). The staining procedures and the concentrations of cells and MAbs were carried out according to Becton Dickison Monoclonal Antibody Source Book. 10,000 viable cells per sample were analysed on a FACS can (Becton Dickinson) using four decade log signal amplification. All viable cells were gated by exclusion of propidium iodide (1 μg/ml). The data were analysed with Lysis II software (Becton Dickinson).
Cell-mediated cytotoxicity assay
The cytotoxicity of the culturedγδ T cells against K562 and Daudi were measured by LDH-release assay in 24 h as previously described18. A commercially available enzymatic test kit for LDH (Kyokuto Pharmaceutical Industries, Co., Tokyo) was used. The assays were performed automatically with a laboratory processor (Biomek 1000, Beckman Instruments, Inc., USA). The cytotoxicity to SQ-5 was measured by crystal violet staining (CV) method. Briefly, SQ-5 cells were seeded into 96-well microculture plates at a density of 100 cells/well with 100 μl MEM containing 10% FBS, and were precultured at 37°C overnight. The SQ-5 cells in 6 control wells were trypsinized for counting before addition of γδT cells. The viable taxget cells were counted with a hematocytometer, then an appropriate number of γδT cells adjusted to the desired E/T ratio were added to each well in 100 ml 5% FBS RHAMa(+) supplemented with 250 U/ml IL-2. The plates were then incubated at 37°C for 24 h in a humidified CO2 incubator. After incubation, the culture medium was discarded and the plates were washed twice with PBS. 200 μl fixing-solution (2% paraformaldehyde, 2% CaC12) per well was added to fix the remaining SQ-5 cells for 1 min. Then, fixing solution was discarded and 100 μl CV staining solution per well was added and stained for 30 min at room temperature. After staining, the plates was washed with water 3 times and dried overnight in air. Then, to each well 200 μl 70% methanol was added to extract the dye. After 1 minute extraction, the absorbance at 540 nm was measured.
Results
Selective proliferation of γδ T cells of healthy donors
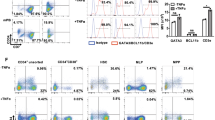
The freshly isolated PBMCs from healthy donors were cultured in the medium containning different concentrations of MEP. At different times, the cell concentrations and the percentages of γδ T cells and αβ T cells were counted. Fig 1, 2, 3 showed some data from a typical donor KA. After 6 d of stimulation, the cell number (Fig 1) and the percentage of γδ T cells (Fig 2A) increased in a dose-dependent manner, and the percentage of αβ T (Fig 2B) cells decreased correspondingly. It indicated that 0.125 mM MEP (commercial) is sufficient to stimulate the proliferation ofγδ T cells (Fig 1, 2). In the culture containing 1 mM MEP (commercial), the percentage of γδ T cells reached 53% which was 23 times greater than that in the control culture containing 250 U/ml IL-2 but without MEP. Additionally, three representatives of the flow cytometric analysis in this experiment were shown in Fig 2C.
The dose response curve of the percentages of γδ and αβ T cells in the cultured PBMC of the donor KA to MEP (commercial).
PBMC were cultured as indicated in Fig 1. The percentages of the γδ and αβ T cells were measured by FACScan as described in Meterials and Methods.
(A), the percentages of γδ T cells are means for duplicated wells.
(B), the percentages of αβ T cells, FACScan are means for duplicated wells.
(C), three representatives of the flow cytometric analysis in this experiment: left, control cultrue without MEP; middle, with 0.5 mM MEP; right, with 2 mM MEP. The cells from the left upper quadrants were identified as γδ T cells and in the right lower quadrants as αβ T cells. The percentage of the cells in the quadrants are indicated.
The effects of MEP (synthesized) on the PBMC of the donor KA.
PBMC were cultured in RHAMa(+) containing 5% autoplasma, 250U/ml IL-2 and with or without 0.1 mM MEP (synthesized). The cells numbers (A), the percentages of γδ T cells (B) and αβ T cells (C) were measured at 6 d of culture respectively. Values are means for duplicated wells.
The time course of MEP action was briefly illustrated in Fig 3. For instance, when the PBMC were cultured in the medium containing 0.1 mM sythesized MEP, the percentage of γδ T cells increased from 2% in freshly isolated PBMC to 54% on d 6, and to 68% on d 9 (Fig 3B), and the percentage of αβ T cells decreased correspondingly (Fig 3C). Furthermore, there was a correlation between the increase of the ratio of γδ T cells and the increase of the cumulative cell number (Fig 3A, 3B).
In Tab 1, the summarized experimental results from 4 healthy donors showed that after stimulation all the percentages of γδ T cells increased, and that the effect of 0.1 mM synthesized MEP was at least as much as that of 1 mM commercial MEP on raising the γδ T cells percentage in 6 d of culture.
Bulk culture of γδ T cells of healthy donors
We found that the higher concentration of MEP were suitable for the bulk culture of γδ T cells. As presented in Fig 4, after 23 d of culture, the cumulative cell number increased from the beginning 5×105 to 5.64 ×108 in the culture containing 1 mM commercial MEP, to 5.78×108 in the culture containing 0.1 mM synthesized MEP. In the culture containing 1 mM synthesized MEP, the cell number was 8.35×108 on d 23 and rose further to 1.68 × 109 on d 26. The percentages of γδ T cells in these cultures were over 70%.
The growth curves of the PBMC of the donor KA in the medium containing higher concentrations of MEP.
PBMC were cultured in RH-AMa(+) containing 5% autoplasma, 250 U/ml IL-2 and the indicated concentrations of MEP. MEP(s): synthesized MEP; MEP(c): commercial available MEP. The cell numbers were counted at indicated time respectively.
Cytotoxic activities of γδ T cells of healthy donors against allogenic tumor cells
The cytotoxic activities of the bulk cultured γδ T cells of donor KA and donor CH against allogenic tumor cell lines were tested respectively. The data were shown in Fig 5. At an E/T ratio of 4, 52% of the K562 cells and 68% of the SQ-5 cells were killed by the γδ T cells of donor CH (Fig 5A). The γδ T cells from donor KA could kill 80% of the Daudi cells and 42% of the SQ-5 cells at an E/T ratio of 3 (Fig 5B).
The cytotoxic activities of γδ T cells against allogenic tumor cells. PBMC were cultured in RHAMa (+) containing 5% autoplasma, 250U/ml IL-2 and 0.1 mM synthesized MEP for above 15 d. The percentages of γδ T cells were above 70%. The killing activities against SQ-5, K562 and Daudi cells were determined as described in Mateials and Method.
A, the PBMC of donor CH.
B, the PBMC of donor KA. Values are means for duplicated wells.
Selective expansion of γδ T cells of cancer patients
With the same culture conditions, we succesfully induced the selective proliferation of γδ T cells from PBMCs of cancer patients. As shown in Tab 2, when the PBMCs of 7 cancer patients were cultured respectively in the medium containing 1 mM MEP (commercial) and 350U/ml IL-2 for 10-14 d, the percentages of γδ T cells rose to about 50-90% which were much higher than that in the control cultures (5%-30%). Moreover, the cumulative cell numbers also increased correspondingly.
For example, in the patient Kang, after 2 w culture, the percentage of γδ T cells rose to 93%, and the cumulative cell number increase by 100 times.
Cytotoxicity of γδ T cells of the cancer patient against autologous tumor cells
We examined the cytotoxicity of the expanded γδ T cells from the PBMCs of cancer patient Opt against autologous endometrium carcinoma. As shown in Fig 6, after cultured in the medium containing 1 mM commercial MEP for 10 d, the percentage of γδ T cells reached 46.14%, and the surviving percentage of tumor cells was 23% at E/T ratio of 6. However, in the control culture, the percentage of γδ T cells was 14.38%, and the surviving percentage of tumor cells was 57% at E/T tatio of 6. Therefore, there was a positive correlation between the increase of γδ T cells and the cytotoxicity against autologous tumor cells.
The cytotoxic activity of γδ T cells against autologous tumor cells. PBMCs of caner patient Opt were cultured in RHAMa(+) containing 5% autoplasma, 250 U/ml IL-2 and with (-•-) or without (-○-) 1 mM MEP (commercial) for 10 d. A, the cytotoxic activity against autologous tumor cells (endometrium carcinoma) was tested with C.V staining method. B. the percentages of γδ T cells and αβ T cells were measured by FACScan: the cells from the left upper quadrants were identified as γδ T cells and in the right lower quadrants as αβ T cells. The percentages of the cells in the quadrants were indicated.
Discussion
With the combination of MEP and IL-2, we established this simple method for the selective expansion of human γδ T cells in vitro. MEP alone is insufficient to induce the selective proliferaton of γδ T cells and IL-2 is required for maintaining the living of γδ T cells in vitro (data not shown). The lower concentrations of IL-2 (200-300 U/ml) are more suitable for the growth of γδ T cells than the higher concentrations (500-1000 U/ml) that are usually used in LAK induction. On the other hand, even when the cultures have been induced to reach a higher percentage of γδT cells (about 80%), it is still necessary to include MEP in the medium, otherwise the number of γδ T cells will decline.
The molecular analysis of human γδ T cell clones demonstrated that the majority of γδ T cells in human peripheral blood express either Vδ2 or Vδ1 gene fragement19. The studies with specific monoclonal antibodies showed that more than 70% of the γδ T cells in human peripheral blood of most adults are Vδ2 subset cells20. The expansion of Vδ2 γδ T cells in blood is thought to be the result of exposure to foreign antigens or superantigens17. The γδ T cells stimulated by MEP expressed VΥ2 / Vδ2 receptors16. According to the above information, the γδ T cells expanded in our culture system are probably Vδ2 subset.
As for the nature of the ligands for the Vδ2 γδ T cells in human peripheral blood, there were several different reports. One author reported that the ligand from mycobacterium tuberculosis (strain H37Rv) lysates is of nature of carbohydrate moeities15. Another two groups of workers demontrated that the ligand is homologous to the proteins of GroEL heat shock protein family13, 14. In another laboratory, four ligands from the same strain of mycobacterium tuberculosis were isolated and partially characterized, one of the which is a 5-triphosphorylated thymidine-containing compound5. Y. Tanaka and S. Sano reported that synthetic alkylphophates, particularly monoethylphosphate (MEP), can mimic small natural ligands of human γδ T cells 16. They further reported that the natural antigens which are produced by mycobacteria and recognized by human VΥ2 / Vδ2-bearing γδ T cells are isopentenyl pyrophosphate derivatives3. In our experiment, the comparison between two kinds of MEP (synthesized and commercial) of their effects on γδ T cells in 4 healthy donors (Tab 1) suggested that some modifications of the ligand in the synthesized MEP might be responsible for its increaseed efficiency in promoting γδ T cells proliferation, as compared to commercial MEP.
Another question is the mechanism by which the ligands activate γδ T cells. The natural ligands were shown to exhibit all hallmarks of bacterial superantigens defined for αβ T cells15. Whether the synthetic ligands act in the same way or not is unknown. One explanation of the synergic action of MEP and IL-2 in our experiments is that γδ T cells express IL-2 receptors preferentially or the affinity of their IL-2 receptors is increased under the stimulation of MEP. However, why the unique function of γδ T cells has so far not been recognized? One reason is that all our present knowledge about T cell recognition and activation are derived from the studies on αβ T cells[i]. Hence, more experiments are required to delineate the action of MEP, IL-2 on γδ T cells in detail, including the functions of the antigen-presenting cells and the other accessory cells.
In order to evaluate the functional state of the expanded γδ T cell in our culture system, we examined the cytotoxic activities of γδ T cells against three allogenic tumor cell lines (Fig 5). Our data are consistent with the results of the other researchers21. Like NK cells, γδ T cells in human peripheral blood mediate the non-MHC-restricted cytolytic effect against many tumor cells. We succeeded in expanding the γδ T cells from the PBMCs of cancer patients (Tab 2), which could obviously kill the autologous tumor cells (Fig 6) and also some allogenic tumor cells (data not shown). We intend to do more experiments to get the pattern in the cytotoxic activities of γδ T cells from different cancer patients against different allogenic tumor cells. It can be envisaged that the cultured γδ T cells may be a new effector for the adoptive immunotherapy of cancer.
With regard to the responsiveness of the PBMCs from different donors, besides the different intensity described in Tab 1 and 2, we observed another kind of phenomenon, which we called γδ T cells-primed type. When the PBMCs of the donors in this type were cultured in the medium containing IL-2 (about 300 U/ml) but without MEP, the percentage of γδ T cells also rose rapidly from below 5% to above 60% with the increase of the cumulative cell number in the culture (data not shown), suggesting that some prior activation in vivo exerted on the γδ T cells. Additionally, γδ T cell growth was accelerated markedly in the medium containing MEP and IL-2 in the later periods of culture (data not shown). Considering the information that most Vδ2 γδ T cells are positive for CD45RO1, a probable marker for immune memory cells, and that there are some contradictory results of the experiments in vivo in an attempt to clarify the way the immune system remembers past encounters with environmental pathogens22, we think that the primed Vδ2 γδ T cells expansion in our culture system might provide a model in vitro to help the study of the mechanism of immunological memory.
References
Haas W, Pereira P, Tonegawa S . Gamma / Delta Cells. Annu Rev Immunol 1993; 11:637–85.
Viney JL, Diannda L, Roberts S J, et al. Lymphocyte proliferation in mice cogenitally deficient in T-cell receptor αβ+ cells. Proc Natl Acad USA 1994; 91:11948–52.
Tanaka Y, Morita CT, tanaka Y, Nieves E, Brenner MB . Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 1995; 375:155–8.
Ferrick DA, Schrenzel MD, Muvania T, Hsieh B, Ferlin WG, Lepper H . Differential Production of interferon-γ and interleukin-4 in response to Th1 and Th2-stimulating pathogens by γδ T cells in vivo. Nature 1995; 373:255–7.
Constant P, Davodeau F, Peyrat MA, et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 1994; 264:267–70.
Monbaerts P, Arnoldi J, Russ F . Tonegawa S, Kaufnann SHE . Different roles of αβ and γδ T cells in immunity against an intracellular bacterial pathogen. Nature 1993; 365:53–6.
Li W, Poberts SJ, Viney JL, et al. Immunoglobin synthesis and generalized autoimmunity in mice congenitally deficient in αβ T cells. Nature 1994; 369:654–8.
Sciammas R, Johnson RM, Sperling AI, et al. Unique antigen recognition by a herpesvirus- specific TCR-γδ cell. J Immunol 1994; 152:5392–7.
Zocchi MR, Ferrarini M, Migone N, Casorati G . T-cell receptor Vd gene usage by tumour reactive γδ T lymphocytes infiltrating human lung cancer. Immunology 1994; 81:234–9.
Schild H, Mavaddat N, Litzenberger C, et al. The nature of Major Histocompatibility Complex Recognition by γ/δ T cells. Cell 1994; 76:29–37.
Rock EP, Sibbald PR, Davis MM, chien YH, et al. CDR3 length in antigen-specific immune receptors. J Exp Med 1994; 179:323–8.
Raulet DH . Antigen for γδT cells. Nature 1989; 339:342–3.
Sturm E, Braaknab E, Fisch P, Vreugdenhil RJ, Sondel P, Bolhuis RLH . Human Vγ9/Vδ T cell receptor-γδ T lymphocytes show specificity to Dandi Burkitt's lymphoma cells. J Immunol 1990; 145:3202–8.
Fisch P, Malkovsky M, Kovats S, et al. Recognition by Human Vγ9/V2δ T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science 1990; 250:1269–73.
Pfeffer K, Schoel B, Plesmila N, et al. A lectin-binding, protease-resistant mycobacterial ligand specifically activates Vγ9+ human T cells. J Immunol 1992; 148:575–83.
Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human γδ T cells. Pro Natl Acad Sci USA 1994; 91:8175–9.
Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J Exp Med 1990; 171:1597–1612.
Kawai K, et al. Additive effects of antitumor drugs and lymphokine- activated killer cell cyto- toxic activity in tumor cell killing determined by lactate- dehydrogenase- release assay. Cancer Immunol. Immunother 1992; 35:225–9.
Casorati G, Libero GD, Lanzavecchia A, Migone N . Molecular analysis of humanγ/δ+ clones from thymus and peripheral blood. J Exp Med 1989; 170:1521–5.
Bottino C, Tambussi G, Ferrini S, et al. Two subsets of human T lymphocytes expressing γ/δ antigen receptor are identificable by monoclonal antibodies directed to two distinct molecular forms of the receptor. J Exp Med 1988; 168:491–505.
Fisch P, Malkovsky M, Braakman E, et al. γ/δ T cell clones and natural killer cell clones mediate distinct patterns of non-major Histocompatibility Complex-restricted cytolysis. J Exp Med 1990; 171:1567–79.
Matzinger P . Memories are of this? Nature 1994; 369:605–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, S., Oki, A., Ohno, T. et al. Selective proliferation of human γδ T cells in vitro. Cell Res 6, 177–187 (1996). https://doi.org/10.1038/cr.1996.19
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/cr.1996.19
Keywords
This article is cited by
-
Human ovarian cancer stem-like cells can be efficiently killed by γδ T lymphocytes
Cancer Immunology, Immunotherapy (2012)