Abstract
Full grown oocytes derived from Bufo Bufo gargarizans rearing at high temperature environment (24 °C), never underwent GVBD after progesterone treatment. No p34cdc2 H1 kinase activity was detected in the oocytes after progesterone stimulation or OA microinjection; Western blotting analysis showed that the level of p34cdc2 and p33 in the oocytes are significantly lower than those in the oocytes derived from the hibernating toads (below 10 °C). 35S-Met incorporation analysis showed that when the oocytes were incubated at 6 °C, synthesis of about thirty different polypeptides was promoted or induced, including p34cdc2 and some other p13sucl-binding proteins. All these results indicated that a low temperature environment is essential for the oocytes of Bufo Bufo gargarizans to express and store some cell cycle drivers and its regulators, and to gain the maturation competence. These results will also provide a new clue for explaining the molecular mechanisms why gametogenesis of some organisms depends on a relative low temperature and how to maintain the geographical distribution of some animals.
Similar content being viewed by others
Introduction
Temperature is one of the main factors that effects the reproduction cycle and gametogensis of some organisms. The gametes at different developmental stages of gametogenesis depend on somewhat different tepmperature. For example, the spermatogenesis of some mammals is completed during their hibernation period1, the spermatogenesis in testes of scrotal mammals needs to have a local temperature in scrotum lower than that of the body temperature2. It is also well known that fullgrown oocytes derived from Bufo Bufo gargarizans rearing at high temperature environment (28 °C), designated as HTE-oocytes, or before entering hibernation state never acquire the competence of maturation. In contrast, the full-grown oocytes derived from the toad hibernating at low temperature environment (<10 °C), designated as LTE-oocytes, can respond to progesterone stimulation and resume meiotic devision3, 4. In vitro experiments also have shown that the HTE-oocytes acquired the maturation competence after a certain period of incubation at low temperature environment (below 10 °C), and the process is independent to any gonadotropic hormones or sex hormones stimulation5. Further analysis revealed that there were some early events of maturation process (such as the decrease of the endogeneous cAMP level, etc.) which took place in HTE-oocytes after progesterone treatment, but neither maturation promoting factor (MPF) activity nor the ability to amplify MPF activity6 were detectable. These results indicated that there may be some deficiency in components necessary for the late events of oocytes maturation process6.
A stock of inactive p34cdc2/ cyclin B (inactive pre-MPF) was produced during the course of oogenesis in oocytes, and required only a post-translational modification for the conversion of this inactive form into an active one. At the onset of meiosis, it was the phosphatase (cdc25) which activates MPF and assures its autoamplification by dephosphorylating Tyrl5 and most probably Thrl4 of its catalytic subunit “p34cdc2”7. In the present study, we showed that the deficiency in MPF and some of its regulators may be the main cause of the incapability of HTE-oocytes to gain the maturation competence. Moreover, we found that the synthesis of p34cdc2 and some other cell cycle regulators are promoted by low temperature in the HTE-oocytes.
Materials and Methods
Materials
Bufo Bufo gargarizans were obtained from JiangSu Province in December, some of them were kept in cold room (4 °C) to maintain the full -grown oocytes sensitive to progesterone stimulation. The others were reared in a high temperature environment (28 °C) all over the year in order to make oocytes inert to progesterone treatment, which means that oocytes of this kind will never undergo germinal vesicle breakdown (GVBD) after progesterone stimulation.
Progesterone, glycerophosphate, PMSF, leupeptin, PKI (20 peptide inhibitor) were purchased from Sigma. γ-32P-ATP (3000ci/mmol) and ECL Western Blotting System were obtained from Amersham. H1 histone, DTT were obtained from Boehringer. p13 agarose was obtained from Santa Cruz. Okadaic acid (OA) was brought from Life and Technologies.
Full-grown (about 1.8 mm in diameter) oocytes used for microinjection with OA were selected from the toad's ovary fragment, ovarian membrane and follicular membrane around oocytes were divested manually with a pair of microforceps under a stereoscope. The nude oocytes were incubated in Ringer's solution at proper temperature for the experiment. Full-grown oocytes for the extraction of proteins were free from their ovarian and follcular membranes by collagenase treatment.
The rabbit antiserum against the conserved amino acid sequence of p34cdc2 “EGVPSTAIREISLLKEC”- keyhole limpet hemocyonin (KLH) complex (anti-PSTAIRE) and antiserum against the conserved amino acid sequence of Y-box binding protein “VRNGYGFINRNDSKEDVC” -KLH complex were prepared by our laboratory according to the methods described in Molecular Cloning (second edition)8. The HRP conjugated goat anti-mouse or anti-rabbit lgG were obtained from Promega Company.
Extraction of oocytes proteins and electrophoresis
oocyte proteins were extracted by homogenizing the oocytes in EB (80mM glycerophosphate, 50 mM NaF, 20 mM naphthylphosphate, 20 mM EGTA, 15 mM MgC12, 1 mM ATP, 1 mM DTT, 300 μM PMSF, 3μg/ml leupeptin, 20 mM HEPES, pH 7.5), followed by ultracentrifugation (100,000g, 1 h, 4 °C). The supernatants were treated with sample buffer containing 10 % mercaptoethanol for 3 min at 100 °C, and then analysed by SDS-PAGE in 12.5 % gel.
Western blotting analysis
Proteins separated by SDS-PAGE were transferred to nitrocelleluse membrane by electro-blotting The membrane was rinsed in Tris-buffered saline (TBS: 20mM tris-HC1, 150 mM NaC1, pH7.5), blocked with 5 % non-fat dry milk in TBST (20 mM tris-HC1, 150 mM NaC1, 0.1% Tween 20) for 30 min, and then incubate with primary antibody for 1 h; after washed three times (5 min each) with TBST; the membrane was incubated with a 1:1000 HRP-conjugated goat anti-rabbit IgG or 1:2500 anti-mouse IgG in TBST for 30 min. Following further wash with TBST for three times, drained the excess buffer from the washed membrane, incubated in the ECL detection (the mixture of detection solution 1 and detection solution 2) for 1 min at room temperature, drained off excess detection reagent and wrapped the membrane in Saran Wrap. Then exposed for 15 seconds to 1 h in film cassette on hyperfilm (Amersham).
H1 kinase assays
The 20μl of protein extracts from oocytes were mixed on ice with equal volume of 40 mM HEPES-NaOH (pH 7.3) consisting 10 mM NaEGTA, 0.2 mg/ml histone H1, 0.2 mM ATP, 0.25 μci γ-32P-ATP and 10 μM cAMP-dependent protein kinase inhibitor. After incubation for 10 min at 22°C, the reactions were terminated by treated with SDS-PAGE sample buffer. Samples were electrophoresed on 12.5 % polyacrylamide gel. After visualization of H1 histone by staining with coomassie blue, 32P incorporation was quantitated by scintillation counting of excised gel pieces or exposed for autoradiography.
Isolation of p34cdc2 with p13suc1 agarose beads
The nude HTE-oocytes or LTE-oocytes were incubated at 28°C or 6°C respectively in Ringer's solution for 6 h, and then were transferred to the Ringer's solution containing 35S-Met (1 mci/ml) incubated at the respective temperature environment for another 6 h. The oocytes were washed three times in Ringer's solution, and then homogenizing in EB and centrifugated 10 min (20,000 g, 4 °C). 50μl sepharose CL-6B (pre-washed with EB) was added into the tubes containing 1 ml the extracts (2 mg/ml proteins), and were kept under constant rotation at 4 °C for 1 h. After centrifugation at 1000 g for 2 min, the supernatant was collected then 100μ1 p13 beads (pre-washed with EB) were added and the tubes were kept under constant rotation at 4 °C for another 6 h. After centrifugation at 1000 g for 2 min of EB after removed of the supernatant. The beads were treated with SDS-PAGE sample buffer and the solubilized proteins were electrophoresed on 12.5 % polyacrylamide gel, stained with coomassie blue, dried, and then detected by autoradiography.
Microinjection of okadaic acid
75 nl of different dilutions of OA were prepared from a stock solution of 2.5 mM OA/DMSO in 10 mM HEPES (pH 7.4) buffer containing 1 mg/ml BSA and 5 mM DTT, and were microinjected into the oocytes at the animal hemisphere near the equator and incubated at 18°C in Ringer's solution for further analysis.
Results and Discussion
No p34cdc2 H1 kinase activity were detected in the HTE-oocytes after progesterone or OA stimulation
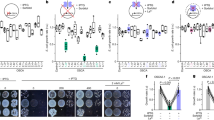
It has been verified that the HTE -oocytes can not undergo GVBD after progesterone treatment and do not manifest any activity of MPF autoamplification5, 6. Results from the H1 kinase activity assay revealed that a high level of p34cdc2 H1 kinase activity was induced in the progesterone treated LTE-oocytes, but no trace of p34cdc2 H1 kinase activity was detected in the HTE-oocytes before or after progesterone treatment (Fig 1). So this may be the reason why GVBD could not take place in the HTE-oocytes after the hormone stimulation.
It is well known that the p34cdc2 H1 kinase is activated by a phosphatase (cdc25), which is negatively regulated by another phosphatase PP2A. Moreover, the activity of PP2A can be strongly inhibited by OA. So, cdc25 can be activated by suppressing PP2A with OA, which in turn can activate p34cdc2 H1 kinase7, 9. As OA can activate MPF indirectly, OA can undoubtly induce GVBD of HTE-oocytes if their MPF subunits(p34cdc2 and cyclin B) and cdc25 are present normally.
We found that 50 % effective dose (ED50) OA to induce GVBD in LTE-oocytes by microinjection was 47.5 femtomoles (Fig 2a), and it took about 6 h to induce 100% of LTE-oocytes maturation by injecting 74 femtomoles of OA (Fig 2b). While the HTE-oocytes never underwent GVBD even injecting 75 femtomoles of OA, and no p34cdc2 H1 kinase activity was detected in HTE-oocytes (Fig 3), indicating that at least one of the subunits of MPF (P34cdc2 and cyclin B) or cdc25 may be in a deficient state.
The levels of p34cdc2 H1 kinase activity in oocytes after OA microinjection
Lane 1, control, LTE-oocytes injected with HEPES buffer without OA;
Lane 2, LTE-oocytes injected with HEPES buffer containing OA;
Lane 3, LTE-oocytes treated with progesterone;
Lane 4, control, HTE-oocytes injected with HEPES buffer without OA;
Lane 5, HTE-oocytes injected with HEPES buffer containing OA;
Lane 6, HTE-oocytes treated with progesterone.
The levels of p34cdc2 and its homologues are different in LTE- and HTE-oocytes
Western blotting analysis with monoclonal antibody specifically against p34cdc2 and antiserum against PSTAIRE peptide showed that the amounts of p34cdc2 and its homologue “p33” in LTE-oocytes are five and ten times more than those in HTE-oocytes (Fig 4, data of densitometric analysis not shown). It was reported that the low level of p34cdc2 may account for their inability to resume meiosis upon exposure to OA in GVBD-incompetent mouse oocytes. After two days in vitro culture, the granulosa cell-free GVBD- incompetent mouse oocytes accumulate p34cdc2 autonomously and become progressively more responsive to OA10.
Enormous p34cdc2 and the other cell cycle control factors are expressed and stored during oogenesis in oocytes which will be necessary for meiosis and early embryonic cell division11. No report has mentioned about the proper amount of p34cdc2 required for oocytes to resume in meiosis. Even there was little amount of p34cdc2 in HTE-oocytes, nor any p34cdc2 H1 kinase activity could be detected in HTE-oocytes treated with progesterone or OA. Our further experiments showed that the enriched p34cdc2 -cyclin B complexes (after precipitation with the p13suc1 beads) from both LTE-and HTE-oocytes (from a total number of 40 oocytes) contained only low level of H1 kinase activity (unpublished data), indicating that the quality of p34cdc2-cyclin B complexes existed in HTE-oocytes is similar to that in LTE-oocytes. Thus, the low level of p34cdc2 and the deficiency of cdc25 (a phosphatase to activate pre-MPF7 seem to be the main causes that HTE-oocytes were unable to gain the maturation competence.
It is also interesting to note that a p34cdc2 homologue with a molecular mass of 33.5 kD (called p33.5) was only expressed in HTE-oocytes, and was not recognized by monoclonal antibody specific to p34cdc2 (Fig 5), but its functions is still unclear. On the other hand, it was reported that there are some faster migrating forms of p34cdc2 or p33 as recognized by the antiserum to PSTAIRE in the mature oocytes of goldfish and starfish12, 13. In the similar manner, we found that there are two faster migrating forms (p32 and p31) appeared in the mature oocytes of Bufo bufo gargarizans (Fig 4). These results indicated that the appearance of different p34cdc2 homologues are affected by different external environmental conditions.
The synthesis of p34cdc2 and some p13suc1 binding proteins are promoted by low temperature in HTE-oocytes
To assess the effect of temperature on the rates of synthesis of p34cdc2 and its homologue in oocytes, HTE-oocytes or LTE-oocytes were incubated at 28°C or 6°C in Ringer's solutions with 35S-Met (1 mci/ml) for 6 h, and then performed the precipitation analysis with p13sue1 agarose respectively. It was found that the synthesis of some kind of peptides, including a peptide with the molecular mass around 34 kD which we strongly suspected to be corresponded to p34cdc2 or its homologue, and two other peptides with the molecular mass of 45 kD and 24 kD (cold shock protein 24, csp24), were promoted by low temperature only in HTE- oocytes (Fig 6). We also found that csp24 was a protein which bound to p13suc1, but did not bind to p34cdc2 /cyclin B complex (unpublished data), indicating csp24 may play an important role in MPF activation or in MPF localization in oocytes.
The synthesis rates of p34cdc2 and some p13suc1 binding proteins in HTE-oocytes and LTE-oocytes incubated at different temperature.
35S-Met incorporated proteins of oocytes were precipitated with p13suc1 agarose, and then subjected to SDS-PAGE, and exposed for autoradiography. The extracts of HTE-oocytes were incubated at 28°C (lane 1) and 6°C (lane 2), and those of LTE-oocytes were incubated at 28°C (lane 3) and at 6°C (lane 4).
It was reported that there are some new and enhanced synthesis of cold shock proteins in cultured amphibian cells15, in winter wheat14 and Escherichia coli etc.. One of the major cold shock proteins in E.coli, called cspA, shares a sequence similarity with the nucleic acid binding domain (cold shock domain) of Y-box binding proteins (such as FRGY1 etc.) in eukaryotic cells16. Similar to Y-box binding proteins, cspA can promote the expression of the genes which possess CCAAT element in their promoters. In addition, most of the low temperature inducible promoters contained CCAAT element in the E.coli17, 18. We were surprised to know that there are two copies of CCAAT elements in the promoters of human cdkl gene and Xenopus cdk2 gene19, 20. Meanwhile, we found that one of the Y-box binding proteins called “p54” was highly expressed in LTE-oocytes (Fig 7) and positively correlated with the high level of p34cdc2 and p33 in these oocytes, suggesting that p54 may be one of'the factors involved in the process of the synthesis of the p34cdc2 and its homologue induced by low temperature.
The Y-box binding protein p54 is expressed mainly in LTE-oocytes. Results obtained from immunoblotting with antiserum against VRNGYGFINRNDSKEDVC peptide (a conserved sequence in the cold shock domain of Y-box binding protein). The extract of HTE-oocytes (lane 1) and the extract of LTE-oocytes (lane 2).
All these findings make a more reasonable explanation on the mechanism concerning the acquisition of maturation competence in oocytes which depends on hibernation: the low temperature as a external signal induces or enhances the expression of some cold shock proteins (such as p54 and csp24), and then the Y-box binding proteins as transcriptional or translational regulators, promoting the oocytes to express and store some proteins (such as p34cdc2 and p33) related to oocytes maturation competence. Furtherneve, these results will also help us to understand the dependence of spermatogenesis in scrotal mammals on a relative low temperature, the mechanism of vernalization of plants for sprouting and flowering, and also the molecular base of the geographical distribution of the toad which is restricted to the region north to the 23o north latitude and east to the 100o east longitude in China.
References
Barnes BM, Kretzmann M, Licht P, Zucker I . Reproduction development of hibernation ground squirrels. In: “Living in the cold”, edited by Heller HC, Published by Elsevier Science Publishing CO., Inc 1986: p245–52.
Waiters GMH, Setchell BP . Physiology of mammalian testis. In: “Marshall Physiology of Reproduction”, edited by Lamming GE, published by Chuchill Livingstone, 1990: vol. 2, p1–100.
Tchou S, Wang YL . L'hibernation, facteur determinant de la maturetion ovulaire chez le crapaud (Bufo bufo asiatius), Scientia Sinica 1963; 12:1161–4.
Tchou S, Wang YL . La succession d'ovogenese et l'impossibilite de maturation ovulaire chez le crapaud femelle elevee dans le milieu a haute temperature pendant toute une annee. Scientia Sinica 1963; 12:1165–68.
Zhao JX, Zhu G, Hsu KG, Wang YL, Tso Jk . In vitro studies on changes of the response to progesterone stimulation of ovarian oocytes obtained from toads reared at high temperature. Acta Biol Exper Sinica 1988; 21(2):168–77.
Zhu G, Hsu KG, Wang YL, Gu Z, Tso Jk . Experimental analysis of mechanism concerning the environmental temperature effect on acquisition of capability in toad oocytes to resume meiotic division. Acta Biol Exper Sinica 1993; 26(4):469–482.
Matten WT, Vande Woude GF . Cell cycle control and early embryogenesis: Xenopus laevis oocyte maturation and early embryonic cell cycles. Seminar in Developmental Biology 1994; 5:173–81.
Sambrook J, Fritsch EF, Maniatis T . Molecular cloning: A laboratory Mannual. 1989; 2nd ed. Cold Spring Harbor, N.Y.. Cold spring Laboratory.
Goris J, Hermann J, Hendix P, Ozon R, Merlevede W . Okadaic acid: a specific protein phosphatase inhibitor induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Lett 1989; 245:91–4.
Chesnel F, Eppig JJ . Synthesis and accumulation of p34cdc2 and cyclin B in mouse oocytes during acquisition of competence to resume meiosis, Mol Reprod and Develop 1995; 40:503–8.
Lohka MJ, Hayes MK, Maller JL . Purification of maturation promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci USA 1988; 85:3009–13.
Kajiura H, Yamashita M, Katsu Y, Nagahama Y . Isolation and characterization of goldfish cdc2, a catalytic component of maturation promoting factor, Develop Growth and Differ 1993; 35(6):647–54.
Ookata K, Hisanaga S, Okano T, Tachibana K, Kishimoto T . Relocation of distinct subcellular localization of p34cdc2 -cyclin B complex at meiosis reintiation in starfish oocytes, EMBO J 1992; 11(5):1763–72.
Danyluk J, Rassart E, Sarhan F . Gene expression during cold and heat shock in wheat. Biochem Cell Biol 1991: 69:383–91.
Ketola-Pirie CA, Atkinson BG . Cold and heat shock induction of new gene expression in cultured amphibian cell. Can J Biochem 1983; 61:462–71.
Wistow AP . Cold shock and DNA binding. Nature 1993; 334:823–24.
Qoronfleh MW, Debouck C, Keller J . Identification and charaterization of novel low temperature inducible promoters of Escherichia coli. J Bacteriol 1992; 174(24):7902–9.
Wolffe AP . Structure and functional properities of evolutionarily ancient Y-box family of nucleic acid binding proteins. Bioessay 1993; 16:245–51.
Dalton S . Cell cycle regulation of human cdc2 gene. EMBO J 1992; 11:153–80.
Olive M, Theze N, Philippe M, Le Pennec JP, Lerivray H . Cloning of the Xenopus laevis cdk2 promoter and functional analysis in oocytes and during early development. Gene 1994; 151:81–8.
Author information
Authors and Affiliations
Additional information
Supported by grant No. 39630160 of the National Nature Science Foundation
Rights and permissions
About this article
Cite this article
Lu, J., Gu, Z., Sheng, H. et al. Low temperature induces oocytes p34cdc2 synthesis and accumulation- the acquisition of competence to resume meiosis in toad oocytes. Cell Res 6, 115–124 (1996). https://doi.org/10.1038/cr.1996.13
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/cr.1996.13
Keywords
This article is cited by
-
A novel ubiquitin carboxyl terminal hydrolase is involved in toad oocyte maturation
Cell Research (2002)