Abstract
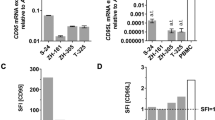
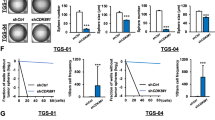
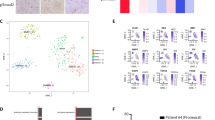
We have previously reported the presence of Dlxin-1, a member of the melanoma antigen gene (MAGE) family, in the brain and showed its function as a cell cycle arrest protein, suggesting that Dlxin-1 may have anti-proliferative functions in rapidly growing tumors. Using the cancer stem cell hypothesis, which attributes the initiation and progression of brain tumors to the cancer-initiating stem cells, we have investigated the role of Dlxin-1 in the glioma stem cells propagated by us as a cell culture system comprising of HNGC-2 cells. Our studies provide evidence about the role of Dlxin-1 as an anti-tumorigenic protein in the highly chemo-resistant glioma stem cells. Next, we show that these anti-proliferative effects are manifested by Dlxin-1 through down regulation of the activities of MMP-2 and MMP-9, and through interaction of Dlxin-1 with its target protein P311 that is involved in glioma cell invasion. In summary, we establish the roles for Dlxin-1, one as an anti-tumorigenic and anti-invasive protein in high-grade gliomas and the other as an inducer of differentiation of glioma stem cells. These two attributes, in conjunction, result in conversion of the drug-resistant brain tumor stem cells to the tumor-attenuated cells that may now be more amenable to effective therapeutic targeting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA 2003; 100: 15178–15183.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401.
Bao SD, Wu QL, McLendon RE, Hao YL, Shi Q, Hjelmeland AB et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–760.
Das S, Srikanth M, Kessler JA . Cancer stem cells and glioma. Nat Clin Pract Neurol 2008; 4: 427–435.
Rebetz J, Tian D, Persson A, Widegren B, Salford LG, Englund E et al. Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma. PLoS ONE 2008; 3: e1936.
Barker PA, Salehi A . The MAGE proteins: emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J Neurosci Res 2002; 67: 705–712.
Williams ME, Strickland P, Watanabe K, Hinck L . UNC5H1 induces apoptosis via its juxtamembrane region through an interaction with NRAGE. J Biol Chem 2003; 278: 17483–17490.
Bertrand MJ, Kenchappa RS, Andrieu D, Leclercq-Smekens M, Nguyen HN, Carter BD et al. NRAGE, a p75NTR adaptor protein, is required for developmental apoptosis in vivo. Cell Death Differ 2008; 15: 1921–1929.
Shiras A, Bhosale A, Patekar A, Shepal V, Shastry P . Differential expression of CD44(S) and variant isoforms v3, v10 in three-dimensional cultures of mouse melanoma cell lines. Clin Exp Metastasis 2002; 19: 445–455.
Shiras A, Chettiar ST, Shepal V, Rajendran G, Prasad GR, Shastry P . Spontaneous transformation of human adult nontumorigenic stem cells to cancer stem cells is driven by genomic instability in a human model of glioblastoma. Stem Cells 2007; 25: 1478–1489.
Chu CS, Xue B, Tu C, Feng ZH, Shi YH, Miao Y et al. NRAGE suppresses metastasis of melanoma and pancreatic cancer in vitro and in vivo. Cancer Lett 2007; 250: 268–275.
Tian XX, Rai D, Li J, Zou C, Bai Y, Wazer D et al. BRCA2 suppresses cell proliferation via stabilizing MAGE-D1. Cancer Res 2005; 65: 4747–4753.
Mosmann T . Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63.
Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987; 47: 3239–3245.
Chung CY, Murphy-Ullrich JE, Erickson HP . Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell 1996; 7: 883–892.
Shi SR, Cote RJ, Taylor CR . Antigen retrieval immunohistochemistry: past, present, and future. J Histochem Cytochem 1997; 45: 327–343.
Shiras A, Sengupta A, Shepal V . Cloning and tissue-specific gene expression studies with Dlxin-1, a newly identified transcriptional activator. Mol Cell Biol Res Commun 2001; 4: 313–319.
Corti S, Locatelli F, Papadimitriou D, Donadoni C, Salani S, Del Bo R et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells 2006; 24: 975–985.
Shiras A, Bhosale A, Shepal V, Shukla R, Baburao VS, Prabhakara K et al. A unique model system for tumor progression in GBM comprising two developed human neuro-epithelial cell lines with differential transforming potential and coexpressing neuronal and glial markers. Neoplasia 2003; 5: 520–532.
Shen WG, Xue QY, Zhu J, Hu BS, Zhang Y, Wu YD et al. Inhibition of adenovirus-mediated human MAGE-D1 on angiogenesis in vitro and in vivo. Mol Cell Biochem 2007; 300: 89–99.
Xia WY, Chen JS, Zhou X, Sun PR, Lee DF, Liao Y et al. Phosphorylation/cytoplasmic localization of p21(Cip1)/(WAF1) is associated with HER2/neu overexpression and provides a novel combination predictor for poor prognosis in breast cancer patients. Clin Cancer Res 2004; 10: 3815–3824.
Ruan S, Okcu MF, Pong RC, Andreeff M, Levin V, Hsieh JT et al. Attenuation of WAF1/Cip1 expression by an antisense adenovirus expression vector sensitizes glioblastoma cells to apoptosis induced by chemotherapeutic agents 1,3-bis(2-chloroethyl)-1-nitrosourea and cisplatin. Clin Cancer Res 1999; 5: 197–202.
Zhang W, Kornblau SM, Kobayashi T, Gambel A, Claxton D, Deisseroth AB . High levels of constitutive WAF1/Cip1 protein are associated with chemoresistance in acute myelogenous leukemia. Clin Cancer Res 1995; 1: 1051–1057.
Li Y, Dowbenko D, Lasky LA . AKT/PKB phosphorylation of p21(Cip/WAF1) enhances protein stability of p21(Cip/WAF1) and promotes cell survival. J Biol Chem 2002; 277: 11352–11361.
Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer 1999; 79: 1828–1835.
Koike A, Kobayashi Y, Takagi T . Kinase pathway database: an integrated protein-kinase and NLP-based protein-interaction resource. Genome Res 2003; 13: 1231–1243.
Koike A, Takagi T . PRIME: automatically extracted protein interactions and molecular information database. In Silico Biol 2005; 5: 9–20.
McDonough WS, Tran NL, Berens ME . Regulation of glioma cell migration by serine-phosphorylated P311. Neoplasia 2005; 7: 862–872.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003; 63: 5821–5828.
Li ZZ, Wang H, Eyler CE, Hjeimeiand AB, Rich JN . Turning cancer stem cells inside out: an exploration of glioma stem cell signaling pathways. J Biol Chem 2009; 284: 16705–11609.
Rich JN, Bao S . Chemotherapy and cancer stem cells. Cell Stem Cell 2007; 1: 353–355.
Ward RJ, Dirks PB . Cancer stem cells: at the headwaters of tumor development. Annu Rev Pathol 2007; 2: 175–189.
Park DM, Rich JN . Biology of glioma cancer stem cells. Mol Cells 2009; 28: 7–12.
Mayes DA, Hu YJ, Teng Y, Siegel E, Wu XS, Panda K et al. PAX6 suppresses the invasiveness of glioblastoma cells and the expression of the matrix metalloproteinase-2 gene. Cancer Res 2006; 66: 9809–9817.
Song T, Wu J, Fang F, Chen FH, Huo L, Zhang MY et al. Correlation analysis between the expression of P21WAF1/CIP1, P16 proteins and human glioma. Clin Exp Med 2008; 8: 151–157.
Kumar PS, Shiras A, Das G, Jagtap JC, Prasad V, Shastry P . Differential expression and role of p21(cip/waf1) and p27(kip1) in TNF-alpha-induced inhibition of proliferation in human glioma cells. Mol Cancer 2007; 6: 42.
Hwang CY, Lee C, Kwon KS . Extracellular signal-regulated kinase 2-dependent phosphorylation induces cytoplasmic localization and degradation of p21(Cip1). Mol Cell Biol 2009; 29: 3379–3389.
Lee S, Helfman DM . Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/cofilin pathway. J Biol Chem 2004; 279: 1885–1891.
Sun X, Liu M, Wei Y, Liu F, Zhi X, Xu R et al. Overexpression of von Hippel-Lindau tumor suppressor protein and antisense HIF-1 alpha eradicates gliomas. Cancer Gene Ther 2006; 13: 428–435.
Thoma CR, Toso A, Gutbrodt KL, Reggi SP, Frew IJ, Schraml P et al. VHL loss causes spindle misorientation and chromosome instability. Nat Cell Biol 2009; 11: 994–U200.
Mariani L, McDonough WS, Hoelzinger DB, Beaudry C, Kaczmarek E, Coons SW et al. Identification and validation of P311 as a glioblastoma invasion gene using laser capture microdissection. Cancer Res 2001; 61: 4190–4196.
Martens T, Laabs Y, Gunther HS, Kemming D, Zhu Z, Witte L et al. Inhibition of glioblastoma growth in a highly invasive nude mouse model can be achieved by targeting epidermal growth factor receptor but not vascular endothelial growth factor receptor-2. Clin Cancer Res 2008; 14: 5447–5458.
Acknowledgements
We sincerely thank Dr Dattatraya Mujumdar, KEM Hospital, Mumbai for providing us with glioma samples, Dr Mahesh M Mandolkar, Dinanath Mangeshkar Hospital, Pune for the histopathology sections, CSIR, Government of India for student fellowships and NCCS, Pune, India for intramural financial support and DBT, Government of India for extra-mural funding support for the research work.
Grant: Grant support provided by Department of Biotechnology, Government of India, New Delhi. The fellowship of EMR, STC, RGK and NK was supported by Council of Scientific and Industrial Research (CSIR), New Delhi, India.
Disclaimer
The manuscript entitled ‘Dlxin-1, a member of MAGE family, inhibits cell proliferation, invasion and tumorigenicity of glioma stem cells’ is original research.
The above mentioned submitted manuscript has not been previously published.
We have not sent this manuscript for publication elsewhere while it is under consideration in Cancer Gene Therapy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Cancer Gene Therapy website
Rights and permissions
About this article
Cite this article
Reddy, E., Chettiar, S., Kaur, N. et al. Dlxin-1, a member of MAGE family, inhibits cell proliferation, invasion and tumorigenicity of glioma stem cells. Cancer Gene Ther 18, 206–218 (2011). https://doi.org/10.1038/cgt.2010.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2010.71
Keywords
This article is cited by
-
Dynamic expression of Mage-D1 in rat dental germs and potential role in mineralization of ectomesenchymal stem cells
Scientific Reports (2022)
-
Complex roles of NRAGE on tumor
Tumor Biology (2016)
-
Prognostic relevance of melanoma antigen D1 expression in colorectal carcinoma
Journal of Translational Medicine (2012)