Abstract
Background:
The one-step nucleic acid amplification (OSNA) assay is a novel molecular method that can detect metastasis in a whole lymph node based on cytokeratin 19 mRNA copy number. This cohort study aimed to establish an OSNA-based nodal staging (pN(mol)) classification for breast cancer.
Methods:
The cohort consisted of 1039 breast cancer patients who underwent sentinel node (SN) biopsy using the OSNA assay. Cutoff value of the SN tumour burden stratifying distant disease-free survival (DDFS) was determined, and predictive factors for DDFS and breast cancer-specific survival (BCSS) were investigated. pN(mol) classification of the SN status was defined as: pN0(mol)(sn), SN negative; pN1mi(mol)(sn), SN positive and tumour burden
Results:
Cutoff value of the SN tumour burden was 2810 copies per μl. Of the 1039 patients, 798, 95, and 146 had pN0(mol)(sn), pN1mi(mol)(sn), and pN1(mol)(sn) status, respectively. Five-year DDFS and BCSS rates were lower for pN1(mol)(sn) patients than for pN1mi(mol)(sn) patients (87.7% vs 98.8%, P=0.001 and 93.1% vs 98.8%, P=0.044, respectively). Multivariate analyses revealed the pN(mol) classification was most significant predictor for DDFS and BCSS.
Conclusions:
The molecular-based pN classification determines the prognosis of breast cancer patients and could guide therapeutic decision making.
Similar content being viewed by others
Main
Axillary lymph node status is one of the most powerful prognostic factors in breast cancer (Fisher et al, 1983). Accurate and reproducible pathological node staging (pN) classification is an important determinant of the prognosis and therapeutic decision making for breast cancer patients. Sentinel lymph node (SN) biopsy has been the standard axillary staging procedure for clinically node-negative patients since the early 1990s (Lyman et al, 2014). To prevent false-negative diagnoses, pathologists began to perform a more detailed evaluation of a fewer amount of lymph nodes, which are most likely to contain metastasis (Giuliano et al, 1995). The intensive examination of SNs resulted in an increase in the detection of low-volume metastases (Weaver et al, 2009).
The Cancer Staging Manual of the American Joint Committee on Cancer (AJCC), 6th edition, in 2002 (Green et al, 2002) classified these low-volume metastases into isolated tumour cells (ITC) and micrometastases. ITC was classified as pN0(i+) with deposits ⩽0.2 mm, and micrometastasis was classified as pN1mi with deposits >0.2 mm to ⩽2 mm. Moreover, in the 7th edition of the AJCC Staging Manual in 2010 (Edge et al, 2010), T1 with lymph node spread confined to micrometastasis (pN1mi) was downstaged from Stage IIA to Stage IB. However, published studies have reported divergent and conflicting results regarding the prognostic significance of ITC and micrometastasis as defined by the AJCC Staging Manual (Patani & Mokbel, 2011; Salhab et al, 2011). These divergent and conflicting results can be attributed to the fact that the AJCC pN classification is based on histopathological findings.
Conventional histopathological examinations are limited in their ability to accurately quantify the total metastatic volume of a lymph node. Even if a node is step-sectioned and histologically evaluated at each cut surface, the information gathered is incomplete, since only a small part of the node is analysed. Furthermore, histopathological examination procedures for SNs are non-standardised, and the inter-observer reproducibility of measuring metastatic tumour volume is low (Cserni et al, 2005).
The one-step nucleic acid amplification (OSNA) assay (Sysmex, Kobe, Japan) was developed to overcome the limitations of histopathological examination of lymph nodes. This assay can assess the whole lymph node and yields the quantitative data in the form of the cytokeratin 19 (CK19) mRNA copy number (Tsujimoto et al, 2007). Calibration and validation studies (Tamaki et al, 2009; Tsujimoto et al, 2007) have provided reasonable evidence that the CK19 mRNA copy numbers detected by the OSNA assay are good estimates of macrometastasis, micrometastasis, and negative, as defined by the AJCC Staging Manual (Edge et al, 2010). We have shown that the OSNA whole-node assay detects more cases of SN metastases, particularly micrometastasis, than conventional histological examinations (Osako et al, 2011b; Osako et al, 2012).
Therefore, the OSNA whole-node assay would enable us to more accurately and reproducibly determine the prognosis of breast cancer patients than the current AJCC pN classification based on histopathological examinations. In order to establish a new molecular-based pN (pN(mol)) classification using the OSNA assay, this retrospective cohort study was designed to determine and validate the prognostic cutoff values of the metastatic tumour burden in the SN, as quantified by the CK19 mRNA copy number.
Patients and methods
Patients
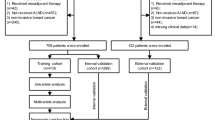
The retrospective cohort included patients with clinically and radiologically node-negative invasive breast cancer who underwent SN biopsy and whose whole SNs were examined using the OSNA assay at the Cancer Institute Hospital (Tokyo, Japan) between April 2009 and June 2011. The exclusion criteria were as follows: (1) SN mapping without the use of a radioisotope tracer, (2) bilateral breast cancer, (3) heterochronous ipsilateral breast cancer recurrence, (4) previous excision of a primary tumour, and (5) neoadjuvant drug therapy. The written general consent was obtained from each of the patients, and this study was approved by the Institutional Review Board of the Cancer Institute Hospital.
The pathological tumour staging (pT) classification was classified according to the 7th edition of the AJCC Staging Manual (Edge et al, 2010). Hormone receptor status and human epidermal growth factor receptor-2 (HER2) status were defined according to the American Society of Clinical Oncology/College of American Pathologists guidelines (Hammond et al, 2010; Wolff et al, 2013). The labelling index value for Ki67 was evaluated by estimating the % of positive nuclei within the areas of highest labelling density.
SN biopsy
Lymphoscintigraphy using 99mTc-phytate was performed one day prior to the surgery, and a vital blue dye, indigo carmine, was injected into the peri-tumoural space or areola at the time of surgery. Before surgery for the primary tumour, the SNs were identified using a hand-held gamma-probe with guidance from staining of the vessels and nodes. Radioactive and/or blue nodes were considered to be SNs and were excised. When the SN(s) were positive, additional axillary lymph node dissection was performed.
OSNA assay
Each of the whole lymph nodes were homogenised with 4 ml lysis buffer solution (Lynorhag; Sysmex) and centrifuged at 10 000 g at room temperature (Tsujimoto et al, 2007). A total of 2 μl supernatant was analysed with an automated molecular detection system, the RD-100i System (Sysmex) and the LynoampBC Kit (Sysmex). The degree of amplification was detected on the basis of a reaction by-product, pyrophosphate. The resultant change in turbidity upon precipitation of magnesium pyrophosphate was then correlated with the CK19 mRNA copy number per μl of the original lysate via a standard curve established beforehand with three calibrators containing different CK19 mRNA copy numbers. Standard positive and negative control samples were used for quality assurance in every assay run. Lymph nodes that exceeded the specified maximum weight of 600 mg were cut into two or more pieces and processed separately.
The number of CK19 mRNA copies per μl in the measurement sample and the 1:10 diluted sample were calculated; the result was determined based on these copy numbers. When the reaction was inhibited in the measurement sample, the copy numbers in the diluted sample were employed for this determination. Lymph nodes with CK19 mRNA <250 copies per μl were considered to be negative, including ITC, and lymph nodes with CK19 mRNA 250–5000 copies per μl or ⩾5000 copies per μl were considered to be equivalent to AJCC micrometastasis or macrometastasis, respectively (Tsujimoto et al, 2007). Tsujimoto et al. determined these cutoff values by measuring CK19 mRNA in 23-mm3-size metastatic tumour tissues and histopathologically positive and negative lymph nodes using the OSNA assay. In their clinical validation study, half of each lymph node was assessed by the OSNA assay and the remaining half was paraffin embedded for three-level histological examination with CK19 immunostaining, and an overall concordance rate between those methods was 98.2% (Tsujimoto et al, 2007).
pN classifications of the SN status
Two pN classifications of the SN status were evaluated: that is, the AJCC pN classification and the new pN(mol) classification. For applying the OSNA assay results to the AJCC pN classification (Edge et al, 2010), each of the SNs was classified into negative, micrometastasis or macrometastasis using the original cutoff values (<250, 250–5000, and ⩾5000 copies per μl, respectively).
For defining the new pN(mol) classification, a cutoff value for the tumour burden in the SN stratifying distant disease-free survival (DDFS) was determined. When more than one SN specimen was examined, the total copy number was considered as the tumour burden. The pN(mol) classification of the SN status was defined as follows: pN0(mol)(sn), SN negative; pN1mi(mol)(sn), SN positive and the total copy number
Detection of non-SN metastasis
Non-SNs in the axillary dissection materials were examined with routine histology or the OSNA assay according to the study period. Between April 2009 and September 2009, non-SN metastasis was detected with single-section histopathology. Between September 2009 and June 2011, each non-SN was examined with the OSNA whole-node assay for clinical research (Osako et al, 2011a; Osako et al, 2013).
Adjuvant treatment and follow-up
After the surgery, the patients received a combination of routine adjuvant treatments according to international standards and the national guideline, based on tumour characteristics, including hormone receptor status, HER2 status, lymph node status, and surgical treatment. Patients were followed-up with a clinical examination, mammography, and ultrasonography.
Statistical analyses
To compare the frequencies of non-SN metastasis, we performed two-sample test for equality of proportions with continuity correction.
DDFS and breast cancer-specific survival (BCSS) were used as prognostic endpoints. DDFS was defined as the period from surgery to distant metastasis of breast cancer, and BCSS was defined as the period from surgery to breast cancer death. The cumulative survival rates were calculated by the Kaplan–Meier method.
To define the new pN(mol) classification, an optimal cutoff value for the tumour burden in the SN was determined according to the maximally selected log-rank statistics analysis. For validating the prognostic impact of the pN(mol) classification on DDFS and BCSS, univariate log-rank tests and multivariate Cox proportional hazards regression models were used. Multivariate analysis was used for the significant factors from the univariate analyses, and the optimal models were selected by Akaike’s Information Criterion. P-values <0.05 were considered to be significant, and the confidence intervals (CI) were set at the 95% level. All statistical analyses were performed with R statistical software (version 3.3.2, http://www.r-project.org/).
Results
Patient characteristics
Between April 2009 and June 2011, 1296 patients with invasive breast cancer underwent SN biopsy using the OSNA whole-node assay, and 1039 of them did not meet the exclusion criteria. The demographic characteristics of the entire cohort are presented in Table 1. Of the 1039 patients, 319 (30.7%) received adjuvant cytotoxic chemotherapy, and 117 (36.7%), 4 (1.3%), 194 (60.8%), and 4 (1.3%) of them received anthracycline-containing regimen alone, taxane-containing regimen alone, both the anthracycline and taxane, and other regimens, respectively. The median follow-up time was 68.3 months (range, 2.0–85.8).
pN(mol) classification of the SN status
The best discriminative cutoff value of the metastatic tumour burden for stratifying DDFS was 2810 copies per μl (Figure 1). Using this cutoff value, of the 1039 patients, 798 (76.8%), 95 (9.1%), and 146 (14.1%) had pN0(mol)(sn), pN1mi(mol)(sn), and pN1(mol)(sn) status, respectively. The demographic characteristics of each category are presented in Table 1.
Non-SN status of SN-positive patients
Apart from one patient with pN1mi(mol)(sn) disease, all of the SN-positive patients underwent additional axillary dissection. Macrometastases in non-SN were more frequently found in pN1(mol)(sn) patients than in pN1mi(mol)(sn) patients (47 out of 146, 32.2% vs 13 out of 94, 13.8%, P=0.002) (Figure 2). However, there was no difference in the frequency of non-SN metastasis (micro- and macrometastasis) between pN1mi(mol)(sn) patients and pN1(mol)(sn) patients (41 out of 94, 43.6% vs 73 out of 146, 50.0%, P=0.40). Regarding the examination method for non-SN, the OSNA assay detected more cases of non-SN micrometastasis than the single-section histology.
The non-SN status of pN1mi(mol)(sn) patients and pN1(mol)(sn) patients according to the examination method for the non-SNs. Abbreviations: SN=sentinel lymph node; OSNA=one-step nucleic acid amplification.
*One patient did not undergo additional axillary dissection; **P<0.01; † difference in the frequency of non-SN metastasis (micro- and macrometastasis).
Distant disease-free survival
Five-year DDFS rates were lower for pN1(mol)(sn) patients than for pN0(mol)(sn) patients (87.7% vs 98.0%, hazard ratio (HR) 6.94 (3.50–13.77), P<0.001) and for pN1mi(mol)(sn) patients (87.7% vs 98.8%, HR 12.95 (1.73–95.00), P=0.001) (Figure 3A). There was no significant 5-year DDFS difference between pN1mi(mol)(sn) and pN0(mol)(sn) patients (98.8% vs 98.0%, HR 0.55 (0.07–5.15), P=0.56).
In the univariate analysis, in addition to the pN(mol)(sn) status, DDFS was significantly related to breast surgery procedure, pT classification, grade, hormone-receptor status, Ki67 labelling index, AJCC pN(sn) classification, positive SN ratio, non-SN status, and adjuvant cytotoxic and endocrine therapies (Table 2). On the other hand, DDFS was not significantly related to age, lymphovascular invasion, HER2 status, and adjuvant anti-HER2 therapy. In the multivariable analysis, pN(mol)(sn) classification, progesterone receptor status, pT classification, and Ki67 labelling index remained significant (Table 3).
Breast cancer-specific survival
Five-year BCSS rates were lower for pN1(mol)(sn) patients than for pN0(mol)(sn) patients (93.1% vs 99.4%, HR 10.06 (3.37–30.02), P<0.001) and for pN1mi(mol)(sn) patients (93.1% vs 98.8%, HR 6.30 (0.80–49.70), P=0.044) (Figure 3B). There was no significant 5-year BCSS difference between pN1mi(mol)(sn) and pN0(mol)(sn) patients (98.8% vs 99.4%, HR 1.70 (0.20–14.54), P=0.63).
In the univariate analysis, in addition to the pN(mol)(sn) status, BCSS was significantly related to pT classification, grade, lymphovascular invasion, hormone-receptor status, Ki67 labelling index, AJCC pN(sn) classification, positive SN ratio, non-SN status, and adjuvant endocrine therapy (Table 2). On the other hand, BCSS was not significantly related to age, breast surgery procedure, HER2 status, and adjuvant cytotoxic and anti-HER2 therapies. In the multivariable analysis, pN(mol)(sn) classification, progesterone receptor status, and pT classification remained significant (Table 3).
Discussion
As far as we know, the present study is the first report to establish a new molecular-based lymph node staging classification for breast cancer without using any histopathological examinations. The new pN(mol) classification is characterised by a total quantification of the metastatic tumour burden in the SN based on the CK19 mRNA copy number using the OSNA whole-node assay, which can more accurately and reproducibly evaluate the metastatic volume than conventional histopathological examinations. Using the pN(mol) classification, pN1(mol)(sn) patients showed a significantly worse prognosis than pN0(mol)(sn) or pN1mi(mol)(sn) patients, and the SN status was the most powerful prognostic factor in early-stage breast cancer.
The prognostic cutoff value was set at 2,810 copies per μl of CK19 mRNA, which is within the range of the tumour burden equivalent to AJCC micrometastasis (250–5000 copies per μl) (Tsujimoto et al, 2007). Therefore, patients with AJCC micrometastasis can possibly be divided into a good prognosis group and a poor prognosis group according to the metastatic volume. However, conventional histopathological examinations are limited in their ability to accurately and reproducibly evaluate the micrometastasis in a lymph node. This may be attributed to the divergent and conflicting results of the prognostic significance of AJCC micrometastasis in previous studies (Salhab et al, 2011). On the other hand, the OSNA assay can accurately and reproducibly evaluate the small metastatic volume, thus the pN(mol) classification could precisely determine patient’s prognosis.
A Spanish group has recently proposed the prognostic cutoff value of 25 000 copies per μl (Peg et al, 2017), which is higher than the cutoff value obtained in the present study (2810 copies per μl). The Spanish group determined the cutoff value by quartering the tumour burdens and testing each of the quartile points for statistical significance. In the present study, however, the optimal cutoff value was more precisely determined by selecting the minimum P-value of all possible cutoff points shown in the Figure 1. Applying the Spanish cutoff value to the Figure 1, this cutoff value is statistically significant (P=8.31e-8), but the cutoff value of 2810 copies per μl is more significant for stratifying patient survival (P=1.18e-11) than the Spanish cutoff value. In addition, another group has recently proposed a similar cutoff value (2150 copies per μl) as the present study for predicting non-SN metastasis (Terrenato et al, 2017). Thus, we believe that our cutoff value can more accurately stratify patient survival than the Spanish cutoff value.
pN1mi(mol)(sn) patients showed similar prognosis to pN0(mol)(sn) patients, even though pN1(mol)(sn) patients showed significantly worse prognosis than pN0(mol)(sn) or pN1mi(mol)(sn) patients. According to the Dutch MIRROR study, micrometastases in regional lymph nodes are associated with a reduced disease-free survival rate among early-stage breast cancer patients who did not receive adjuvant therapy; however, adjuvant therapy improved survival (de Boer et al, 2009). However, because of the present retrospective study design, it is unknown if pN1mi(mol)(sn) patients show worse survival than pN0(mol)(sn) patients without adjuvant chemotherapy, or if pN1mi(mol)(sn) patients intrinsically show similar survival to pN0(mol)(sn) patients despite of adjuvant chemotherapy. Prospective studies are needed to elucidate the prognostic impact of pN1mi(mol)(sn) status.
Using the pN(mol) classification, the SN status can be the most powerful predictive factor for determining both disease-free and cause-specific survival. After the American College of Surgeons Oncology Group Z-0011 randomised trial (Giuliano et al, 2011), additional axillary dissection can be omitted for clinically node-negative patients who have one or two positive SNs and who are receiving adjuvant systemic chemotherapy and breast-conserving surgery with tangential irradiation (NCCN, 2016). Therefore, the pN(mol) classification of the SN status is useful to predict the prognosis of patients who omit additional axillary dissection after positive SN biopsy.
However, the non-SN status in axillary dissection material was not a prognostic factor in the multivariate analysis. This may be because non-SN metastasis, especially macrometastasis, is strongly associated with the SN tumour burden, quantified using the OSNA assay (Osako et al, 2013), and the pN(mol) classification of the SN status can be a cofounding factor for the association between non-SN status and prognosis. However, one study has shown that identifying tumour spread to non-SNs beyond SNs appears to be an important determinant of patient outcome, and is independent of the number of involved nodes (Jakub et al, 2011). We have reported clinical research in which all of the SNs and non-SNs were evaluated by the OSNA whole-node assay without using any histopathological examination (Osako et al, 2011a; Osako et al, 2013). Follow-up of this cohort may clarify the prognostic impact of the non-SN tumour burden and the total axillary metastatic burden.
There are two potential limitations for the establishment of the pN(mol) classification. First, the present study did not directly compare the prognostic influence of the OSNA-based pN(mol) classification with the current histology-based AJCC pN classification. We found that the AJCC pN classification using the OSNA assay results was less significantly associated with prognosis than the pN(mol) classification. Retrospective or prospective studies are necessary for demonstrating the advantage of the pN(mol) classification over the histology-based AJCC pN classification. Second, we adapted the total copy number in the SN for the pN(mol) classification because several previous studies have reported that the total copy number in the SN determines non-SN metastasis (Peg et al, 2017; Terrenato et al, 2017). However, the maximum copy number in the SN (cutoff value of 2500 copies per μl) had a similar prognostic impact as the total copy number (unpublished data). The maximum copy number can possibly be used for the pN(mol) classification as a surrogate for the total copy number.
In conclusion, a new molecular-based lymph node staging classification for breast cancer has been established using the prognostic cutoff value of the SN tumour burden, quantified using the OSNA assay. The SN status using the pN(mol) classification is the most powerful prognostic factor in early-stage breast cancer. The pN(mol) classification could more accurately and reproducibly determine the prognosis than the current pN classification, and may help to guide more precise therapeutic decision making for breast cancer patients.
Change history
07 November 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Cserni G, Bianchi S, Boecker W, Decker T, Lacerda M, Rank F, Wells CA (2005) Improving the reproducibility of diagnosing micrometastases and isolated tumor cells. Cancer 103 (2): 358–367.
de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest PJ, Adang EM, Nortier JW, Rutgers EJ, Seynaeve C, Menke-Pluymers MB, Bult P, Tjan-Heijnen VC (2009) Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med 361 (7): 653–663.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2010) American Joint Committee on Cancer Cancer Staging Manual 7th edn. Springer: New York, NY, USA.
Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, Foster R, Gardner B, Lerner H, Margolese R, Poisson R, Shibata H, Volk H et al (1983) Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 52 (9): 1551–1557.
Giuliano AE, Dale PS, Turner RR, Morton DL, Evans SW, Krasne DL (1995) Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg 222 (3): 394–399.
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305 (6): 569–575.
Green FL, Amendoeira I, Apostolikas N (2002) American Joint Committee on Cancer Cancer Staging Manual 6th edn. Springer: New York, NY, USA.
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28 (16): 2784–2795.
Jakub JW, Bryant K, Huebner M, Hoskin T, Boughey JC, Reynolds C, Degnim AC (2011) The number of axillary lymph nodes involved with metastatic breast cancer does not affect outcome as long as all disease is confined to the sentinel lymph nodes. Ann Surg Oncol 18 (1): 86–93.
Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, Benson AB 3rd, Bosserman LD, Burstein HJ, Cody H 3rd, Hayman J, Perkins CL, Podoloff DA, Giuliano AE (2014) Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology Clinical Practice guideline update. J Clin Oncol 32 (13): 1365–1383.
NCCN (2016) National Comprehensive Cancer Nnetwork clinical practice guidelines in oncology. Breast cancer ver. 2, 2016. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Osako T, Iwase T, Kimura K, Horii R, Akiyama F (2013) Sentinel node tumour burden quantified based on cytokeratin 19 mRNA copy number predicts non-sentinel node metastases in breast cancer: molecular whole-node analysis of all removed nodes. Eur J Cancer 49 (6): 1187–1195.
Osako T, Iwase T, Kimura K, Masumura K, Horii R, Akiyama F (2012) Incidence and possible pathogenesis of sentinel node micrometastases in ductal carcinoma in situ of the breast detected using molecular whole lymph node assay. Br J Cancer 106 (10): 1675–1681.
Osako T, Iwase T, Kimura K, Yamashita K, Horii R, Akiyama F (2011a) Accurate staging of axillary lymph nodes from breast cancer patients using a novel molecular method. Br J Cancer 105 (8): 1197–1202.
Osako T, Iwase T, Kimura K, Yamashita K, Horii R, Yanagisawa A, Akiyama F (2011b) Intraoperative molecular assay for sentinel lymph node metastases in early stage breast cancer: A comparative analysis between one-step nucleic acid amplification whole node assay and routine frozen section histology. Cancer 117 (19): 4365–4374.
Patani N, Mokbel K (2011) Clinical significance of sentinel lymph node isolated tumour cells in breast cancer. Breast Cancer Res Treat 127 (2): 325–334.
Peg V, Sansano I, Vieites B, Bernet L, Cano R, Cordoba A, Sancho M, Martin MD, Vilardell F, Cazorla A, Espinosa-Bravo M, Perez-Garcia JM, Cortes J, Rubio IT, Ramon YCS (2017) Role of total tumour load of sentinel lymph node on survival in early breast cancer patients. Breast 33: 8–13.
Salhab M, Patani N, Mokbel K (2011) Sentinel lymph node micrometastasis in human breast cancer: an update. Surg Oncol 20 (4): e195–e206.
Tamaki Y, Akiyama F, Iwase T, Kaneko T, Tsuda H, Sato K, Ueda S, Mano M, Masuda N, Takeda M, Tsujimoto M, Yoshidome K, Inaji H, Nakajima H, Komoike Y, Kataoka TR, Nakamura S, Suzuki K, Tsugawa K, Wakasa K, Okino T, Kato Y, Noguchi S, Matsuura N (2009) Molecular detection of lymph node metastases in breast cancer patients: results of a multicenter trial using the one-step nucleic acid amplification assay. Clin Cancer Res 15 (8): 2879–2884.
Terrenato I, D'Alicandro V, Casini B, Perracchio L, Rollo F, De Salvo L, Di Filippo S, Di Filippo F, Pescarmona E, Maugeri-Sacca M, Mottolese M, Buglioni S (2017) A cut-off of 2150 cytokeratin 19 mRNA copy number in sentinel lymph node may be a powerful predictor of non-sentinel lymph node status in breast cancer patients. PLoS One 12 (2): e0171517.
Tsujimoto M, Nakabayashi K, Yoshidome K, Kaneko T, Iwase T, Akiyama F, Kato Y, Tsuda H, Ueda S, Sato K, Tamaki Y, Noguchi S, Kataoka TR, Nakajima H, Komoike Y, Inaji H, Tsugawa K, Suzuki K, Nakamura S, Daitoh M, Otomo Y, Matsuura N (2007) One-step nucleic acid amplification for intraoperative detection of lymph node metastasis in breast cancer patients. Clin Cancer Res 13 (16): 4807–4816.
Weaver DL, Le UP, Dupuis SL, Weaver KA, Harlow SP, Ashikaga T, Krag DN (2009) Metastasis detection in sentinel lymph nodes: comparison of a limited widely spaced (NSABP protocol B-32) and a comprehensive narrowly spaced paraffin block sectioning strategy. Am J Surg Pathol 33 (11): 1583–1589.
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31 (31): 3997–4013.
Acknowledgements
We express our appreciation to all of the breast surgeons in the Breast Oncology Center of the Cancer Institute Hospital and all of the pathologists and technicians in the Division of Pathology of the Cancer Institute. This work was supported in part by a Grant-in-Aid for Young Scientists (B) (No. 21791264) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology and a Research Grant from the Foundation for the Promotion of Cancer Research in Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
FA has received consulting fee from Sysmex Corporation paid to the Cancer Institute. The remaining authors declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Osako, T., Iwase, T., Ushijima, M. et al. A new molecular-based lymph node staging classification determines the prognosis of breast cancer patients. Br J Cancer 117, 1470–1477 (2017). https://doi.org/10.1038/bjc.2017.311
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.311
Keywords
This article is cited by
-
Prognostic impact and possible pathogenesis of lymph node metastasis in ductal carcinoma in situ of the breast
Breast Cancer Research and Treatment (2019)
-
Performance of a new system using a one-step nucleic acid amplification assay for detecting lymph node metastases in breast cancer
Medical Oncology (2019)
-
Elucidation of inhibitory effects on metastatic sentinel lymph nodes of breast cancer during One-Step Nucleic Acid Amplification
Scientific Reports (2018)