Abstract
Hedyotis hedyotidea has been used in traditional Chinese medicine for the treatment of autoimmune diseases. However, the mechanisms underlying for the effect remain unknown. We previously showed that, among 11 compounds extracted from H hedyotidea, betulin produced the strongest suppressive effect on T cell activation. Here, we examined the hepatoprotective effects of betulin against acute autoimmune hepatitis in mice and the mechanisms underlying the effects. Freshly isolated mouse splenocytes were stimulated with concanavalin A (Con A, 5 μg/mL) in the presence of betulin, the cell proliferation was assessed with CSFE-dilution assay. Mice were injected with betulin (10, 20 mg·kg−1·d−1, ip) for 3 d. One hour after the last injection, the mice were injected with Con A (15 mg/kg, iv) to induce acute hepatitis. Blood samples and liver tissues were harvested at 10 h after Con A injection, and serum transaminase levels and liver histopathology were detected; serum levels of proinflammatory cytokines, hepatic T lymphocyte ratios, and functional statuses of conventional T and NKT cells were also analyzed. Betulin (16 and 32 μmol/L) dose-dependently suppressed the proliferation of Con A-stimulated mouse splenocytes in vitro. In Con A-challenged mice, preinjection with betulin (20 mg·kg−1·d−1) significantly decreased the levels of proinflammatory cytokines IFN-γ, TNF-α and IL-6, and ameliorated liver injury. Furthermore, pretreatment with betulin (20 mg·kg−1·d−1) significantly inhibited the Con A-induced activation of NKT and conventional T cells, and decreased production of proinflammatory cytokines IFN-γ, TNF-α and IL-6 in these two cell populations. Betulin has immunomodulatory effect on overly activated conventional T and NKT cells and exerts hepatoprotective action in mouse autoimmune hepatitis. The findings provide evidence for the use of H hedyotidea and its constituent betulin in the treatment of autoimmune diseases.
Similar content being viewed by others
Introduction
Hedyotis hedyotidea (H hedyotidea) belongs to the family Rubiaceae and is widespread in southern China. The entire H. hedyotidea plant is used in Chinese folk medicine to treat various diseases1,2 such as cold, cough, gastroenteritis, heatstroke, hepatitis, rheumatoid arthritis, and herpes zoster. Moreover, it is also used in several prescribed herbal formulations used for the treatment of autoimmune diseases, such as Niu-Bai-Teng-He-Si-Miao-Tang for acute rheumatoid arthritis3 and Fu-Fang-Niu-Bai-Teng-Tang for acute hepatitis treatment4.
Despite the traditional use of H hedyotidea in autoimmune diseases, limited studies have been made to identify its anti-inflammatory potential and related mechanism. Several crude/uncharacterized extracts of H hedyotidea have been used to evaluate anti-inflammatory effects associated with the reduction of xylene-induced ear swelling and capillary permeability5. Unfortunately, that study did not present any experimental results concerning the active constituents. The lack of knowledge of the active constituents of H hedyotidea, together with the fact that we have recently identified betulin from among eleven H hedyotidea-derived compounds as having the strongest suppressive effect on T cell activation6, prompted us to examine the effects of betulin on liver injury to decipher the mechanism responsible for the effects of H hedyotidea, a traditional treatment for hepatic diseases.
Betulin, a pentacyclic triterpenoid compounds, was originally isolated from H hedyotidea. It possesses a broad spectrum of pharmacological activities, including anti-tumor7, anti-viral8, anti-inflammatory9, and anti-atherosclerotic plaque10 activities. Recently, Quan11 and Wan12 have reported that betulin and its derivative are effective against alcoholic and non-alcoholic fatty liver disease. These reports suggest that betulin does have a hepatoprotective effect against liver injury. However, the mechanism involved remains unclear, thus limiting the use of betulin and H hedyotidea in the treatment of hepatic autoimmune injury. Therefore, in this study, we sought to elucidate whether and how betulin attenuates hepatitis and/or contributes to the hepatoprotection of H hedyotidea in a concanavalin A (Con A)-induced mouse model of acute hepatitis.
Materials and methods
Plant material and extraction
H hedyotidea was collected from Guangxi Province, China in June 2012. The plant was identified and authenticated by Prof Jia-chun CHEN from the Huazhong University of Science and Technology. A voucher specimen (No 20120611) was deposited at the Laboratory of Pharmacognosy of Huazhong University of Science and Technology. Compounds were extracted and isolated from H hedyotidea as previously described6. Owing to the limited amount of extracted betulin, we obtained additional betulin commercially for in vivo study. The betulin from the H hedyotidea plant and the commercially available betulin had the same structure13.
Reagents
Commercial betulin was purchased from Tauto Biotech Co, Ltd (purity ≥ 98%, Cat E-0164, CAS 473-98-3, Shanghai, China). Concanavalin A type IV (Con A, Cat C2272), carboxyfluorescein diacetate succinimidyl ester (CFSE, Cat 21888) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Cat M5655) were obtained from Sigma (St Louis, MO, USA). A cytometric bead array (CBA) kit for mouse T helper (Th)1/Th2/Th17 cytokines was obtained from BD Biosciences (Cat 560485, San Diego, CA 92121, USA). Alanine aminotransferase (ALT, Cat C009-2) and aspartate aminotransferase (AST, Cat C010-2) microplate assay kits were acquired from the Jiancheng Bioengineering Institute (Nanjing, China). GolgiStop brefeldin A solution (BFA, Cat 00-4506-51), IC fixation buffer (Cat 00-8222-49) and 10×permeabilization buffer (Cat 00-8333-56) were procured from eBioscience, Inc (San Diego, CA 92121, USA). The following monoclonal antibodies (mAbs) were used for cell surface marker staining: FITC-conjugated anti-CD3 (Cat 11-0031, eBioscience), APC/Cy7-conjugated anti-CD3 (Cat 100222, BioLegend) and CD8 (Cat. 100714, BioLegend), APC-conjugated anti-CD8 (Cat 17-0081, eBioscience), PE-Cy7-conjugated anti-CD4 (Cat 25-0041, eBioscience), and PE-conjugated anti-CD69 (Cat 12-0691, eBioscience). APC-conjugated mCD1d/PBS57 tetramers were provided by the National Institutes of Health (NIH) tetramer core facility (30082, USA). The following mAbs against intracellular cytokines were obtained from eBioscience: anti-interferon-γ (IFN-γ, Cat 11-7311), anti-tumor necrosis factor-α (TNF-α, Cat 12-7321), and anti-interleukin-4 (IL-4, Cat 25-7042).
Experimental animals
C57BL/6J mice (8–10 weeks old, 18–24 g) were obtained from the Hubei Research Center of Laboratory Animals (Wuhan, China) and maintained under controlled conditions (22 °C, 50% humidity, 12 h light/dark cycle, with lights on at 7:00 AM). Food and water were provided ad libitum throughout the experimental period. The mouse care and experimental protocols conducted in this study were approved by the Huazhong University of Science and Technology Animal Care and Use Committee.
Proliferation assay
Spleens from C57BL/6J mice were homogenized with syringe plungers to prepare single cell suspensions and passed through 0.1-mm sterile nylon mesh. After erythrocyte depletion, purified splenocytes were resuspended in 0.1% BSA-PBS and labeled with 2 μmol/L CFSE at a density of 3×106 cells/mL for 8 min in a 37 °C incubator. Splenocyte viability was examined by trypan-blue exclusion on a hemocytometer, and in all cases cell viability was higher than 95%. The CFSE-labeled splenocytes were seeded at 3×105 cells/well in 96-well flat-bottom plates (Costar, Cambridge, MA) containing 200 μL RPMI-1640 medium with 10% heat-inactivated fetal bovine serum (FBS, complete medium) plus various concentrations of betulin (0–32 μg/mL) in the presence of Con A (5 μg/mL). The plates were incubated in a humidified chamber at 37 °C and 5% CO2. After 72 h, the CFSE signal of gated splenocytes was detected by using an LSR II flow cytometer (Becton Dickinson, San Jose, CA, USA).
In vitro toxicity assay
One hundred microliters of freshly isolated splenocytes was seeded at 3×105 cells/well in 96-well flat-bottom plates and treated with 100 μL of various concentrations of betulin (0–32 μg/mL, with no more than 0.1% dimethyl sulfoxide, DMSO) in RPMI-1640 complete medium. After cells were cultured for 68 h, MTT was added at a final concentration of 5 mg/mL, and the plates were incubated for another 4 h. Cell-free supernatants were removed, and 150 μL of DMSO was added to the wells. The optical density was detected at 492 nm with a microplate reader (Tecan GENios, Austria). The experiment was repeated three times.
The L02 normal human hepatocyte line was grown in Dulbecco's modified Eagle's medium (DMEM) with 10% FBS and 1% penicillin/streptomycin at a density of 4×104 cells/mL in 96-well flat-bottom plates. The toxicity of betulin on L02 cells was measured by using the above-mentioned MTT assay.
Hepatitis induction and treatment
To evaluate the effects of betulin on acute fulminant hepatitis, the mice were randomly divided into 6 groups. The groups and corresponding treatments are listed in Table 1. The administration time and betulin dosage were determined according to our preliminary experimental results.
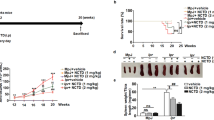
For the survival assay, one Con A challenge and one betulin treatment (Con A+BT20) group (7 mice for each group) were established to monitor the prolonged effects of betulin for 72 h.
Blood samples were collected from the orbital venous plexus under mild pentobarbital anesthesia at 10 h after the Con A injection, and the serum was separated and stored at -80 °C until cytokine and aminotransferase levels were measured. Later, mice were sacrificed by cervical dislocation under mild pentobarbital anesthesia, and livers were harvested, and the weight, lymphocyte subset and histopathology were determined. To evaluate the activation and cytokine production of T and NKT cells, mice were sacrificed 8 h after the Con A injection.
Isolation of hepatic mononuclear cells
Hepatic mononuclear cells (MNCs) were isolated as described by Watarai et al14. Briefly, each mouse liver was first perfused with Hank's balanced salt solution to eliminate blood until the liver become pale. Liver tissues were removed and pressed through a 70 μm cell strainer (Cat 352350, BD, USA). The liver cell suspensions were collected and resuspended in RPMI 1640 medium. Hepatic MNCs were purified by density gradient centrifugation in a 38% Percoll solution (Cat 17-0891-01-1, GE Healthcare, USA).
Flow cytometry analysis
For extracellular staining, cells (1×106) were transferred into FACS tubes and stained with specific surface marker mAbs for 30 min on ice. For intracellular staining, cells were cultured with BFA in the presence or absence of PMA (50 ng/mL) and ionomycin (500 ng/mL) for 4 h. Cells were first stained for surface markers, then fixed with IC fixation buffer. Fixed cells were permeabilized with 1× permeabilization buffer and then stained for the following intracellular cytokines: IFN-γ, TNF-α and IL-4. Data were acquired via FACSVerse™ and analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Serum transaminase assessment
Serum aminotransferase levels (ALT and AST) were determined using commercially available kits according to the manufacturer's instructions.
Serum cytokine assay
The levels of IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-10, and IL-17A in the serum 10 h after Con A challenge were examined with a CBA kit for mouse Th1/Th2/Th17 cytokines according to the manufacturer's protocols.
Histological examination
Liver samples were fixed in 4% formaldehyde, dehydrated in a graded series of alcohol and embedded in paraffin wax. Four-micron-thick sections were cut and stained with hematoxylin-eosin (H&E) and observed by three pathologists in a blinded manner. The extent of the pathology was scored from 0 (no pathology) to 3 (severe pathology) as previously described15.
Statistical analysis
Data are presented as the mean±SEM. Statistical analyses were performed with one-way ANOVA by using Prism 6.0 (GraphPad Software, San Diego, CA, USA). P<0.05 was considered to be statistically significant.
Results
Betulin suppresses the proliferation of Con A-stimulated splenocytes
The betulin isolated from H hedyotidea was confirmed to be identical to the structure previously described by nuclear magnetic resonance (NMR) spectra and mass spectra (MS)6,13 (Figure 1A). Detailed parameters for the spectra are listed in Supplemental Table 1. A total of 12.6 mg of betulin was obtained from 27 kg of crude plant material. The purity of betulin (≥98.5%) was determined via high performance liquid chromatography with a UV detector. To investigate whether betulin contributes to the anti-inflammatory effect of H hedyotidea, a Con A-induced splenocyte proliferation assay was carried out in the presence of betulin. In response to Con A stimulation, mouse splenocytes strongly proliferated. However, betulin attenuated Con A-stimulated splenocyte proliferation in a dose-dependent manner (Figure 1B). To further determine the toxicity of betulin, splenocytes and L02 human normal liver cells were incubated with varying concentrations of betulin (2–32 μg/mL) for 72 h. The results showed that betulin had little toxic effect on either splenocytes or L02 cells at the tested concentrations (supplemental Figure 1). These observations demonstrated that betulin inhibited the proliferation of Con A-stimulated splenocytes and showed little toxicity at a concentration range of 2–32 μg/mL.
Inhibitory effect of betulin on the proliferation of Con A-stimulated splenocytes. (A) Chemical structure of betulin isolated from Hedyotis hedyotidea. (B) Splenocytes from C57BL/6J mice were stimulated with 5 μg/mL Con A in the presence of betulin (BT) with indicated concentration. After 72 h culture, splenocyte proliferation was determined via CFSE-dilution assay. Bar graph depicts the percentages of proliferated cells. Data are expressed as mean±SEM from three independent experiments (6 mice per group). **P<0.01 vs Con A control.
Betulin ameliorates Con A-induced liver injury in mice
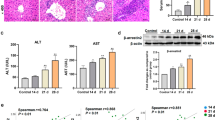
Next, we evaluated the effects of betulin on Con A-induced hepatitis in vivo. Because plasma transaminase is a valid indicator of the severity of hepatic injury, we first examined serum ALT and AST levels in the Con A-challenged mice. As expected, markedly elevated serum levels of ALT and AST were observed (Figure 2A, 2B). Pretreatment with betulin significantly inhibited the increase in serum ALT and AST levels induced by the Con A challenge. In addition, the serum transaminase levels of the betulin control (20 mg/kg) mice were similar to those of the normal control mice (Figure 2A, 2B), thus suggesting little hepatic toxicity of betulin in vivo.
Protective effect of betulin on Con A-induced liver injury. Betulin was first dissolved in DMSO, further diluted to the indicated concentration with PBS. Betulin 10 mg/kg (BT10), 20 mg/kg (BT20) or solvent (DMSO-PBS) was injected ip into mice once daily for 3 d. One hour after the last injection, a total of 15 mg/kg Con A was injected iv to induce acute hepatitis. The mice without Con A injection served as controls. Serum ALT (A) and AST (B) levels were measured at 10 h after Con A injection. (C) Liver tissues were harvested at 10 h post Con A administration, and the tissue sections were stained with H&E. One representative tissue staining from indicated group was shown. The arrows indicated necrotic areas or infiltrated leukocytes (×100). (D) The severity of liver injury was scored as shown in Materials and methods. (E) The ratio of liver/body weight was calculated. (F) Effect of betulin on Con A-induced mortality. Values were mean±SEM. Data are pooled from three independent experiments (7 mice per group). N, normal control; S, solvent control. **P<0.01 vs normal control (N). #P<0.05, ##P<0.01 vs Con A.
To further confirm the hepatic protective effects of betulin, H&E stained liver sections were evaluated for histopathology. In the Con A challenge group, the liver sections exhibited severe signs of liver damage characterized by numerous infiltrating leukocytes and edematous, necrotic hepatocytes [Figure 2C (Con A)]. Consistently with the lower transaminase levels, the liver sections of the betulin-treated mice showed significant protection against the histological signs of hepatic injury [Figure 2C (Con A+BT20)]. Moreover, little hepatic inflammation was found in the normal, solvent and betulin control groups, thus indicating that solvent [Figure 2C (S)] and betulin [Figure 2C (BT20)] had few side effects on the liver. To quantify the histopathological changes, three individuals scored the results in a blinded manner, and the scores were subjected to ANOVA analysis. The results showed lower histopathological scores in the betulin-treated group relative to the Con A challenge group (Figure 2D). Furthermore, betulin significantly attenuated the Con A-induced hypertrophy of liver tissue, as determined on the basis of the liver/body weight ratio (Figure 2E). In addition, treatment with betulin also increased the long-term survival rate relative to that of the Con A challenge group (Figure 2F). Together, these results indicated that pretreatment with betulin provides significant protection against Con A-induced hepatic damage.
Betulin reduces serum levels of IFN-γ, TNF-α, and IL-6 in mice with hepatitis
Several lines of evidence suggest that IFN-γ16,17, TNF-α18,19, IL-420, and IL-1721,22 promote hepatic damage, whereas IL-1023 protects the liver from injury, and IL-618 has varying effects depending on the stage of Con A-induced hepatitis at which it is present. To ascertain whether betulin interfered with the systemic levels of these cytokines, we examined the serum levels of IFN-γ, TNF-α, IL-2, IL-4, IL-6, IL-10, and IL-17 by using a CBA kit. In the Con A challenge group, we found that only the levels of the proinflammatory cytokines IFN-γ, TNF-α, and IL-6 were dramatically increased relative to those in the normal control mice. Betulin ameliorated the Con A-induced cytokine production, because the betulin-treated mice showed markedly decreased circulating levels of IFN-γ, TNF-α, and IL-6 (Figure 3A–3C). There were no significant differences in the serum levels of IL-4, IL-2, IL-17, or IL-10 among the groups (Figure 3D–3G).
Effect of betulin on serum proinflammatory cytokines in Con A challenged mice. Serum samples were collected at 10 h after Con A injection. Serum levels of IFN-γ (A), TNF-α (B), IL-6 (C), IL-4 (D), IL-2 (E), IL-17 (F), and IL-10 (G) were quantified by CBA kit. Bar graphs depict the concentrations of indicated cytokines from different groups. Values were presented as mean±SEM. Data shown are pooled from three separate experiments (7 mice per group). **P<0.01 vs normal control (N). ##P<0.01 vs Con A.
Betulin abolishes Con A-induced activation and inflammatory cytokine production by NKT cells
Activated T cells18,24, including NKT25 cells, contribute to the Con A-induced development and progression of hepatic inflammation. Pentacyclic triterpene compounds are well known to exert anti-inflammatory effects through targeting macrophages, oxidative stress mediators (ie, superoxide dismutase, prostaglandins, cyclooxygenase-2 or nitric oxide)26,27. To investigate whether betulin, a pentacyclic triterpene compound, has an effect on T cells, T cell subsets were analyzed 10 h after Con A injection. In the Con A challenge group, compared with the untreated control group, the percentages of hepatic NKT cells and conventional CD4+ T cells were significantly decreased (Figure 4A, 4C, 4E, 4F), whereas the percentage of hepatic conventional CD8+T cell was increased (Figure 4D, 4F). Notably, betulin treatment partially rescued hepatic NKT cell depletion, because the betulin-treated mice, compared with the Con A challenge mice, showed an increased hepatic NKT ratio (Figure 4A, 4E). Activation-induced NKT cell death has been proposed to be the cause for the selective depletion of hepatic NKT cells after Con A challenge28. Here, we found that betulin treatment decreased CD69 expression on NKT cells (Figure 5A), thus suggesting that betulin restored the NKT cell ratio by inhibiting Con A-induced NKT cell activation, given that CD69 is a surface marker for T cell or NKT cell activation. In addition, betulin also down-regulated the secretion of TNF-α and IFN-γ from NKT cells after Con A challenge in vivo (Figure 5B, 5C). Both cytokines are known to play proinflammatory roles during Con A-induced hepatitis. Therefore, the inhibition of NKT cell activation and the production of proinflammatory cytokines might contribute to the hepatoprotective effects of betulin.
Effect of betulin on hepatic T cell proportion in Con A challenged mice. Ten hours after Con A injection, mice were sacrificed and liver mononuclear cells (MNCs) were isolated. Cells were stained with mAbs against CD3, CD4 and CD8 as well as CD1d-tetramer, and analyzed by flow cytometry. (A–D) Bar graphs depict the percentages of CD3+CD1d-tetramer+ NKT cells (A), CD3+CD1d-tetramer— T cells (conventional T cells) (B), CD3+CD1d-tetramer—CD4+T cells in conventional T cells (C) and CD3+CD1d-tetramer–CD8+ T cells in conventional T cells (D) from different groups. (E) Representative dot plots show the percentages of NKT cells and conventional T cells. (F) CD3+CD1d-tetramer—T cells were analyzed for the expression of CD4 and CD8. Mean±SEM. n=3 separate experiments (7 mice per group). *P<0.05, **P<0.01 vs normal control (N). #P<0.05 vs Con A.
Effect of betulin on activation and proinflammatory cytokine secretion of hepatic NKT cells. Mice were sacrificed, and liver MNCs were isolated at 8 h after Con A injection. (A) CD3+CD1d-tetramer+ NKT cells were analyzed by flow cytometry for CD69 expression. Bar graph (left pannel) depicts percentages of CD69+ NKT cells from indicated groups. Histogram with numbers (right pannel) shows the mean fluorescence intensity (MFI) of CD69 on NKT cells from normal control (N, dash line), Con A (tinted area) and Con A+BT20 (black line) groups. (B and C) Hepatic MNCs were cultured for 4 h in the presence of GolgiStop BFA. IL-4, IFN-γ, and TNF-α producing NKT cells (CD3+CD1d-tetramer+) were analyzed via flow cytometry. (B) Bar graphs depict the mean±SEM of the percentages of indicated cytokines producing NKT cells from different groups. (C) Representative dot plots show the percentages of IFN-γ+(upper row) and TNF-α+ (lower row) cells within CD3+ CD1d-tetramer+ NKT cells from indicated group. Data are pooled from three independent experiments (6 mice per group). **P<0.01 vs normal control (N). #P<0.05, ##P<0.01 vs Con A.
Betulin decreases the activation and inflammatory cytokine secretion of hepatic conventional T cells in Con A-challenged mice
Next, we compared the activation and functional status of hepatic conventional T cells in the Con A challenge and betulin treatment groups. We observed a robust activation of conventional T cells in Con A challenge group, which was characterized an increase in CD69 expression on CD3+CD1d-tetramer– cells. Both conventional CD4+ and CD8+ T cell subsets showed elevated CD69 expression in response to Con A. In the betulin-treated mice, the CD69 expression levels in both populations were markedly decreased, thus indicating that betulin also inhibited the Con A-induced activation of conventional T cells (Figure 6A–6C). Moreover, betulin strongly decreased the secretion of TNF-α and IFN-γ by CD4+ T cells (Figure 6D, 6E). However, the compound did not significantly inhibit Con A-induced cytokine production by CD8+ T cells.
Effect of betulin on activation and proinflammatory cytokine secretion of hepatic conventional T cells. Mice were sacrificed, and hepatic MNCs were prepared at 8 h after Con A injection. CD3+CD1d-tetramer− conventional T cells were analyzed for CD69 expression by flow cytometry. Bar graphs (left pannel) represent the percentages of CD69-positive cells in total conventional T cells (A), CD4+ T cells (B) or CD8+ T cells (C). Histograms with numbers (right Pannel) show the MFI of CD69 on indicated T cell subsets from normal control (N, dash line), Con A (tinted area) and Con A+BT20 (black line) groups. (D and E) Hepatic MNCs were cultured in medium with GolgiStop alone for 4 h. Intracellular staining for IL-4, IFN-γ, and TNF-α was performed, and cells were analyzed by flow cytometry. (D) Bar graphs depict the percentages of indicated cytokine producing cells in the conventional T cell subsets. (E) Dot plots show IFN-γ and TNF-α expression by total T (upper row) and CD4+ T cells (lower row). All the values are shown as mean±SEM and pooled from three independent experiments (6 mice per group). *P<0.05, **P<0.01 vs normal control (N). #P<0.05, ##P<0.01 vs Con A.
PMA and ionomycin are usually used as non-specific activators of polyclonal T cells and stimulate T cells to produce maximal cytokine levels in vitro. Interestingly, after PMA/ionomycin stimulation, the NKT cells and conventional T cells from the betulin-treated mice showed cytokine expression levels similar to those from Con A challenge mice, thus suggesting that the compound did not affect the potential cytokine production capacity of any T cell subset (supplemental Figure 2).
Discussion
Autoimmune hepatitis is a common clinical syndrome with complicated pathological mechanisms. Natural extracts from plants used in Chinese folk medicine have various bioactivities and immunomodulatory effects and provide a promising path for autoimmune hepatitis treatment. Previous studies have identified a large number of substances derived from those plants; these substances have been shown to possess therapeutic potential and low toxicity for treatment of multiple diseases29,30. For example, the species Hedyotis, which is used in folk medicine, provides an abundant source of natural products that are beneficial to hepatitis4.
Con A-induced acute hepatitis in mice is a well-characterized model for studying the pathogenesis of human hepatic diseases and the screening of hepatoprotective drugs24. In Con A-induced hepatitis, the inflammatory response is responsible for liver damage and is mainly driven by proinflammatory Th1 cytokines. High levels of proinflammatory cytokines such as TNF-α18,19 and IFN-γ16 have been shown to promote the progress of this disease. In addition, neutralizing or knocking out the secretion of these cytokines leads to animal resistance to Con A-induced hepatic damage. In the present study, we examined the circulating levels of seven cytokines by using a CBA. Although IL-4, IL-2, and IL-17 have been reported to promote inflammation in Con A-induced hepatitis, we did not observe elevation of these cytokines after Con A injection. Each cytokine is secreted with specific kinetics in response to Con A31. For example, after Con A challenge, circulating TNF-α levels reached the peak first and rapidly decrease; IL-2 and IL-4 reach a peak after approximately 2–4 h and then rapidly decrease; IFN-γ rises slowly, but this peak is long lasting; IL-6 reaches a peak after approximately 3 h and then decreases to a lower level after 6 h18,31. Ten hours after Con A injection, we found that Con A challenge resulted in a burst of IFN-γ, TNF-α, and IL-6 secretion (Figure 3A–3C), results consistent with those from previous reports32. Betulin treatment significantly decreased the levels of IFN-γ, TNF-α, and IL-6 in the serum, thus suggesting that betulin ameliorates the severity of inflammation by suppressing the secretion of the proinflammatory cytokines, particularly Th1-type cytokines.
Accumulating evidence suggests that CD4+ T cells18,31 and NKT cells25,28 are both critical in Con A-induced liver damage. After Con A challenge, polyclonal T cells are directly activated independently of TCR-mediated recognition; this activation is followed by the secretion of a large amount of Th1-type cytokines. Concurrently, hepatic NKT cells are also rapidly activated and secrete Th1 and Th2 type cytokines, such as IFN-γ and IL-4. NKT cells are abundant in the liver33,34, and most NKT cells are CD4 single positive (CD3+CD4+) or double negative (CD3+CD4–CD8–). Activated NKT cells contribute to both the innate and adaptive immune responses. These effects not only defend against diverse pathogens and carcinoma but also lead to tissue damage in inflammation and autoimmune disease. Thus, regulating T and NKT cell activation can be beneficial in the treatment of inflammatory diseases. In the present study, both NKT and conventional T cells, the major participating cell populations in the injured liver, were robustly activated by Con A (Figure 5A and 6A–6C). This activation was significantly attenuated by betulin treatment. The administration of betulin decreased the enhanced expression of CD69 on NKT and conventional T cells (Figure 5A and 6A–6C). Therefore, the inhibition of T and NKT cell activation is one of the anti-inflammatory properties of betulin.
In addition to secreting cytokines, NKT cells also directly participate in perforin/granzyme- and/or Fas/Fas ligand (FasL)-mediated cytotoxicity. The cytotoxicity of NKT cells not only induces hepatic damage but also results in the apoptotic elimination of NKT cells from the liver during Con A challenge28. Our results confirmed the rapid disappearance of activated NKT cells in the Con A-challenged liver (Figure 4A, 4E). Although we did not detect the expression of FasL on NKT cells, the lower CD69 expression level together with the restored NKT cell ratio suggested that the activation-induced cell death (AICD) of NKT cells was abolished in the betulin-treated mice.
Although NKT cells act as the predominant effector cells in Con A-induced hepatitis, the depletion of NKT cells begins as early as 4 h after Con A administration28. Thus, it is the NKT cell-derived proinflammatory cytokine polarizing Th1-biased response rather than NKT cell-mediated cytotoxicity that plays a much more important role in the latter phase of inflammation induced by Con A. Many studies have attempted to target the production of proinflammatory cytokines for autoimmune disease treatment. Nakaya and colleagues35 have reported that SOCS3 in T and NKT cells negatively regulates cytokine secretion and decreases Con A-induced liver injury. Our results showed that betulin down-regulated cytokine production by NKT cells and conventional T cell after Con A challenge (Figure 5B, 5C, and 6D, 6E). Although betulin treatment was observed to abolish the Con A-induced excessive production of inflammatory cytokines in all T cell subsets, it did not impair their potential cytokine production capacities. Because betulin has been reported to inhibit production of pro-inflammatory cytokines by decreasing the activation of nuclear factor-κB (NF-κB)36 and/or activating the STAT337 signaling pathway during the inflammatory response, similar mechanisms may be responsible for the anti-inflammatory effects of betulin observed in this study.
In conclusion, pretreatment with betulin inhibited NKT and conventional T cell activation, and decreased production of proinflammatory cytokines in these two cell populations. These results provide evidence for the immunomodulatory activity of betulin and provide support for the popular use of H hedyotidea and its betulin constituent in the treatment of T cell-dependent autoimmune diseases.
Abbreviation
ConA, concanavalin A; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; CFSE, carboxyfluorescein diacetate succinimidyl ester; AIH, acute induced hepatitis; CBA, cytometric bead array; ICS, intracellular cytokine staining; PMA, phorbol myristate acetate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MNC, mononuclear cell; NKT, natural killer T; DMSO, dimethyl sulfoxide.
Author contribution
Yong-qin ZHOU, Rui DOU, Xiao-sheng TAN, and Tian-tian ZHANG performed the experiments. Yong-qin ZHOU, Rui DOU, and Xiu-fang WENG analyzed the data. Yong-qin ZHOU and Xiu-fang WENG drafted the article. Xiu-fang WENG, Jin-bo FANG, and Xiong-wen WU contributed to con¬ception and design, and revised the paper.
References
Peng J, Feng X, Liang X . Iridoids from Hedyotis hedyotidea. Phytochemistry 1998; 47: 1657–9.
Hu X, Zhang S, Liu S, Xuan L . New anthraquinone and iridoid glycosides from the stems of Hedyotis hedyotidea. Helvetica Chim Acta 2011; 94: 675–85.
Jun H, Lihua L, Xiaoyan C, Hui H . Effect of Si Miao Tang containing Hedyotis hedyotidea on acute gouty arthritis. Zhejiang J Tradit Chin Med 2012; 47: 815.
Renjian W . Initial effect of Fu Fang Hedyotis hedyotidea Tang cure twenty cases acute infectious hepatitis. Guangxi J Tradit Chin Med 1978; 1: 16–7.
Yanfen C, Shuhong T, Jiezhen Y, Chaoyan Y, Xiaorong Y, Shuiqing C . Screening of anti-inflammatory and analgesic effective parts of Hedyotis hedyotidea. Tradit Chin Drug Res Clin Pharmacol 2012; 23: 17–9.
Tiantian Z, Shasha G, Junjie H, Yongqin Z, Jiewen Z, Xiaogang W, et al. Chemical constituents from stems of Hedyotis hedyotidea and their immunosuppressive activity. China J Chin Mat Med 2015; 40: 2357–62.
Li XD, Zhang YJ, Han JC . Betulin inhibits lung carcinoma proliferation through activation of AMPK signaling. Tumour Biol 2014; 35: 11153–8.
Shikov AN, Djachuk GI, Sergeev DV, Pozharitskaya ON, Esaulenko EV, Kosman VM, et al. Birch bark extract as therapy for chronic hepatitis C-A pilot study. Phytomedicine 2011; 18: 807–10.
Sami A, Taru M, Salme K, Jari YK . Pharmacological properties of the ubiquitous natural product betulin. Eur J Pharm Sci 2006; 29: 1–13.
Tang JJ, Li JG, Qi W, Qiu WW, Li PS, Li BL, et al. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab 2011; 13: 44–56.
Quan HY, Kim DY, Kim SJ, Jo HK, Kim GW, Chung SH . Betulinic acid alleviates non-alcoholic fatty liver by inhibiting SREBP1 activity via the AMPK-mTOR-SREBP signaling pathway. Biochem Pharmacol 2013; 8X: 1330–40.
Wan Y, Jiang S, Lian LH, Bai T, Cui PH, Sun XT, et al. Betulinic acid and betulin ameliorate acute ethanol-induced fatty liver via TLR4 and STAT3 in vivo and in vitro. Int Immunopharmacol 2013; 17: 184–90.
Zhang T, Ye Q, Feng C, Chen Y . Chemical study on gladiolus gandavensis. Chin J Appl Environ Biol 2007; 13: 635–40.
Watarai H, Nakagawa R, Omori-Miyake M, Dashtsoodol N, Taniguchi M . Methods for detection, isolation and culture of mouse and human invariant NKT cells. Nat Protoc 2008; 3: 70–8.
Yan J, Jie Z, Hou L, Wanderley JL, Soong L, Gupta S, et al. Parenchyma expression of CD40 exacerbates adenovirus-induced hepatitis in mice. Hepatology 2011; 53: 1455–67.
Kato J, Okamoto T, Motoyama H, Uchiyama R, Kirchhofer D, Rooijen NV, et al. Interferon-gamma-mediated tissue factor expression contributes to T-cell-mediated hepatitis through induction of hypercoagulation in mice. Hepatology 2013; 57: 362–72.
Kusters S, Gantner F, Kunstle G, Tiegs G . Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by concanavalin A. Gastroenterology 1996; 111: 462–71.
Mizuhara H, O'Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, et al. T cell activation-associated hepatic injury mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med 1994; 179: 1529–37.
Wolf D, Hallmann R, Sass G, Sixt M, Küsters S, Fregien B, et al. TNF-alpha-induced expression of adhesion molecules in the liver is under the control of TNFR1-relevance for concanavalin A-induced hepatitis. J Immunol 2001; 166: 1300–7.
Jaruga B, Hong F, Sun R, Radaeva S, Gao B . Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol 2003; 171: 3233–44.
Hou L, Jie Z, Desai M, Liang Y, Soong L, Wang T, et al. Early IL-17 production by intrahepatic T cells is important for adaptive immune responses in viral hepatitis. J Immunol 2013; 190: 621–9.
Lafdil F, Wang H, Park O, Zhang W, Moritoki Y, Yin S, et al. Myeloid STAT3 inhibits T cell-mediated hepatitis by regulating T helper 1 cytokine and interleukin-17 production. Gastroenterology 2009; 137: 2125–35.
Erhardt A, Biburger M, Papadopoulos T, Tiegs G . IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology 2007; 45: 475–85.
Tiegs G, Hentschel J, Wendel A . A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest 1992; 90: 196–203.
Kumar V . NKT-cell subsets: promoters and protectors in inflammatory liver disease. J Hepatol 2013; 59: 618–20.
Tsai JC, Peng WH, Chiu TH, Lai SC, Lee CY . Anti-inflammatory effects of Scoparia dulcis L and betulinic acid. Am J Chin Med 2011; 39: 943–56.
Dufour D, Pichette A, Mshvildadze V, Bradette-Hebert ME, Lavoie S, Longtin A, et al. Antioxidant, anti-inflammatory and anticancer activities of methanolic extracts from Ledum groenlandicum Retzius. J Ethnopharmacol 2007; 111: 22–8.
Takeda K, Hayakawa Y, Kaer LV, Matsuda H, Yagita H, Okumura K . Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A 2000; 97: 5498–503.
Wohlfarth C, Efferth T . Natural products as promising drug candidates for the treatment of hepatitis B and C. Acta Pharmacol Sin 2009; 30: 25–30.
Yu Y, Jing JF, Tong XK, He PL, Li YC, Hu YH, et al. Discovering novel anti-HCV compounds with inhibitory activities toward HCV NS3/4A protease. Acta Pharmacol Sin 2014; 35: 1074–81.
Wang HX, Liu M, Weng SY, Li JJ, Xie C, He HL, et al. Immune mechanisms of Concanavalin A model of autoimmune hepatitis. World J Gastroenterol 2012; 18: 119–25.
Torisu T, Nakaya M, Watanabe S, Hashimoto M, Yoshida H, Chinen T, et al. Suppressor of cytokine signaling 1 protects mice against concanavalin A-induced hepatitis by inhibiting apoptosis. Hepatology 2008; 47: 1644–54.
Kita H, Mackay IR, Water JVD, Gershwin ME . The lymphoid liver: considerations on pathways to autoimmune injury. Gastroenterology 2001; 120: 1485–501.
Krawitt EL . Autoimmune hepatitis. New Engl J Med 2006; 354: 54–66.
Nakaya M, Hashimoto M, Nakagawa R, Wakabayashi Y, Ishizaki T, Takada I, et al. SOCS3 in T and NKT cells negatively regulates cytokine production and ameliorates ConA-induced hepatitis. J Immunol 2009; 183: 7047–53.
Zhao H, Liu Z, Liu W, Han X, Zhao M . Betulin attenuates lung and liver injuries in sepsis. Int Immunopharmacol 2016; 30: 50–6.
Zhang SY, Zhao QF, Fang NN, Yu JG . Betulin inhibits pro-inflammatory cytokines expression through activation STAT3 signaling pathway in human cardiac cells. Eur Rev Med Pharmacol Sci 2015; 19: 455–60.
Acknowledgements
The authors would like to thank the NIH tetramer core facility for the CD1d tetramers, Dr Qin YANG of the Institute of Pathology, Tongji Hospital, Huazhong University of Science and Technology, and Senior Engineer Yaqin Wang and Associate Prof Xia WEI of the Department of Histology and Embryology, Medical College of China Three Gorges University for their help and technical assistance in histological examination. This work was supported by the National Natural Science Foundation of China (No 31370885, 31000150, and 81302528) and the 973 Program from the Ministry of Science and Technology of China (No 2013CB530505).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary information is available at the website of Acta Pharmacologica Sinica.
Supplementary information
Supplementary Figure S1
Betulin had little toxic effects on either splenocytes or L02 cells at the tested concentrations. (JPG 377 kb)
Supplementary Figure S2
Betulin did not affect the potential cytokine production capacity of any T cell subset stimulated with PMA and ionomycin. (JPG 665 kb)
Supplementary Table S1
1H NMR (400 MHz) and 13C NMR (100 MHz) data for betulin (CDCl3, J in Hz) (DOC 44 kb)
Rights and permissions
About this article
Cite this article
Zhou, Yq., Weng, Xf., Dou, R. et al. Betulin from Hedyotis hedyotidea ameliorates concanavalin A-induced and T cell-mediated autoimmune hepatitis in mice. Acta Pharmacol Sin 38, 201–210 (2017). https://doi.org/10.1038/aps.2016.102
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2016.102
Keywords
This article is cited by
-
Phytochemistry, pharmacology, and medical uses of Oldenlandia (family Rubaceae): a review
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Immunomodulatory properties of triterpenes
Phytochemistry Reviews (2022)
-
Total flavonoids from Tetrastigma hemsleyanum ameliorates inflammatory stress in concanavalin A-induced autoimmune hepatitis mice by regulating Treg/Th17 immune homeostasis
Inflammopharmacology (2019)