Abstract
Objective:
Feeding intolerance is a common problem in the neonatal intensive care unit (NICU) and some cases might be causally related to atrophic changes in the small bowel mucosa. We speculated that for such patients, feeding tolerance might improve after oral administration of enterocyte growth factors in a sterile, isotonic solution patterned after amniotic fluid.
Study Design:
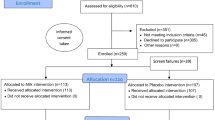
Twenty neonates meeting criteria for feeding intolerance were eligible for this trial. They were randomized to either Group 1 (test solution) or Group 2 (control). Group 1 received 2.5 ml of test solution/kg every 3 h by oral-gastric or nasal-gastric (OG/NG) tube. This was begun when the patient was NPO because of feeding intolerance and continued until 80 ml/k/day of milk feedings were tolerated, or for a maximum of 7 days. Group 2 received a sham OG/NG administration every 3 h, until 80 ml/k/day of milk feedings were tolerated, or for a maximum of 7 days. Only the bedside nurse and the NICU pharmacist were aware which patients received the test solution and which received the sham administrations. The volumes of milk feedings were increased by order of the attending neonatologist and nurse practitioner. The study outcome was enteral calories/kg/day during and for 7 days after the cessation of the treatments.
Results:
Eleven patients were randomized to receive the test solution and nine to receive sham administrations. At study entry, the two groups were not different in gestational age, postnatal age, signs of feeding intolerance or cal/k/day taken enterally during the previous 3 days. The study doses were given for an average of just under 6 days (range, 2 to 7 days). During the week following the administrations, the test solution recipients trended toward more enteral calories. Specifically, they had an increase averaging 78±20.8 cal/k/day more than before the study, whereas the sham recipients had an increase averaging 55.9±33 cal/k/day more than before the study (P=0.05 for a one-sided test and P=0.10 for a two-sided test). The test solution recipients also had a trend toward fewer formula changes than did the sham recipients (P=0.10).
Conclusion:
In this small, randomized, controlled, masked trial, the administration of a sterile, non-caloric, growth factor containing solution patterned after human amniotic fluid was associated with trends that we interpret as reflecting better tolerance of milk feedings. On this basis, we suggest that a phase III efficacy trial should be accomplished, using the present data for sample size calculations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Jadcherla SR, Kliegman RM . Studies of feeding intolerance in very low birth weight infants: definition and significance. Pediatrics 2002; 109: 516–517.
Berseth CL . Feeding methods for the preterm infant. Semin Neonatol 2001; 6: 417–424.
Hernandez G, Velasco N, Wainstein C, Castillo L, Bugedo G, Maiz A et al. Gut mucosal atrophy after a short enteral fasting period in critically ill patients. J Crit Care 1999; 14: 73–77.
Barney CK, Purser N, Christensen RD . Treating feeding intolerance in the NICU by enterally administering a solution patterned after human amniotic fluid. Advan Neo Care 2006; 6: 89–95.
Calhoun DA, Juul SE, McBryde EV, Veerman MW, Christensen RD . Stability of filgrastim and epoetin alfa in a system designed for enteral administration in neonates. Ann Pharmacother 2000; 34: 1257–1261.
Calhoun DA, Richards BE, Gersting JA, Sullivan SE, Christensen RD . G-CSF and Epo stability in amniotic fluid during simulated in vitro digestion conditions. J Pharm Tech 2002; 18: 310–315.
Calhoun DA, Lunø M, Du Y, Christensen RD . Granulocyte colony-stimulating factor is present in human milk and its receptor is present in human fetal intestine. Pediatrics 2000; 105: e7.
Juul S, Joyce A, Zhao Y, Ledbetter D . Why is erythropoietin present in human milk? Studies on erythropoietin receptors on enterocytes of human and rat neonates. Pediatr Res 1999; 46: 263–268.
Juul SE, Christensen RD . Absorption of enteral recombinant human erythropoietin by neonates. Ann Pharmacother 2003; 37: 782–786.
Calhoun DA, Maheshwari A, Christensen RD . Recombinant granulocyte colony-stimulating factor administered enterally to neonates is not absorbed. Pediatrics 2003; 112: 421–423.
Sullivan SE, Calhoun DA, Maheshwari A, Ashmead TL, Aurebach DA, Hudak ML et al. Tolerance of simulated amniotic fluid in premature neonates. Ann Pharmacother 2002; 36: 1518–1524.
Lima-Rogel V, Calhoun DA, Maheshwari A, Torres-Montes A, Roque-Sanchez R, Garcia MG et al. Tolerance of a sterile isotonic electrolyte solution containing select recombinant growth factors in neonates recovering from necrotizing enterocolitis. J Perinatol 2003; 23: 200–204.
Lima-Rogel V, Ojeda MA, Villegas G, Torres-Montes A, Medrano S, Calhoun DA et al. Tolerance of an enterally administered simulated amniotic fluid-like solution by neonates recovering from surgery for congenital bowel abnormalities. J Perinatol 2004; 24: 295–298.
Christensen RD, Havraneck T, Gerstmann DR, Calhoun DA . Enteral administration of a simulated amniotic fluid to very low birth weight neonates. J Perinatol 2005; 25: 380–385.
Condino AA, Barleycorn AA, Lu W, Maheshwari A, Christensen RD, Calhoun DA . Abnormal intestinal histology in neonates with congenital anomalies of the gastrointestinal tract. Biol Neonate 2004; 85: 145–150.
Karnak I, Tanyel F, Muftuoglu S, Unsal I, Cakar N, Buyukpamukcu N . Esophageal ligation: effects on the development of fetal organic systems. Eur J Pediatr Surg 1996; 6: 328–333.
Trahair J, Harding R . Restitution of swallowing in the fetal sheep restores intestinal growth after midgestation esophageal obstruction. J Pediatr Gastroenterol Nutr 1995; 20: 156–161.
Maheshwari A . Role of cytokines in human intestinal villous development. Clin Perinatol 2004; 31: 143–156.
Juul SE . Recombinant erythropoietin as a neuroprotective treatment: In vitro and in vivo models. Clin Perinatol 2004; 31: 129–142.
Calhoun DA, Christensen RD . Hematopoietic growth factors in neonatal medicine: The use of enterally administered hematopoietic growth factors in the neonatal intensive care unit. Clin Perinatol 2004; 31: 169–182.
Acknowledgements
We thank the NICU nurses at McKay Dee Hospital Center for their valuable assistance with this study, and Dr Darlene A Calhoun, Sarasota, Florida, for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website (http://www.nature.com/jp)
Supplementary information
Rights and permissions
About this article
Cite this article
Barney, C., Lambert, D., Alder, S. et al. Treating feeding intolerance with an enteral solution patterned after human amniotic fluid: a randomized, controlled, masked trial. J Perinatol 27, 28–31 (2007). https://doi.org/10.1038/sj.jp.7211609
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7211609
Keywords
This article is cited by
-
Gut priming with bovine colostrum and T regulatory cells in preterm neonates: a randomized controlled trial
Pediatric Research (2021)
-
Standardized feeding regimen for reducing necrotizing enterocolitis in preterm infants: an updated systematic review
Journal of Perinatology (2017)
-
Protective effects of amniotic fluid in the setting of necrotizing enterocolitis
Pediatric Research (2017)
-
Necrotizing enterocolitis in term neonates: data from a multihospital health-care system
Journal of Perinatology (2007)