Boron's unusual properties inspired big advances in chemistry. A compound in which boron binds two carbon monoxide molecules reveals another oddity — the element forms bonds similar to those of transition metals. See Letter p.327
Transition-metal compounds take part in a wide range of reactions and catalytic cycles as a result of the metals' abilities to form complexes with several ligands through a process known as donor–acceptor bonding. Many transition metals can bind several carbonyl ligands (carbon monoxide molecules). By contrast, the main-group elements — those found in groups 1, 2 and 13 to 18 of the periodic table, excluding hydrogen — were for a long time thought not to be able to form complexes that bind more than one carbonyl. Writing on page 327, Braunschweig et al.1 report the synthesis of a compound, known as a borylene adduct, in which an atom of the main-group element boron carries two carbonyl ligands, and which has remarkable similarities to transition-metal carbonyl complexes. This provides striking evidence of donor–acceptor bonding in the chemistry of main-group elements.
Boron forms compounds in which the element exhibits bonding patterns and reactivities not found for other elements. Indeed, two Nobel prizes for chemistry have been awarded for discoveries involving this property of boron. In 1976, William Lipscomb received the award for his work on the structure of boranes (boron–hydrogen compounds), which illuminated problems of chemical bonding2. Three years later, Herbert Brown was recognized for his research into organoboron compounds (sharing the prize with Georg Wittig for his related groundbreaking discoveries in the area of phosphorus chemistry)3. A third Nobel prize may also be partly ascribed to discoveries in boron chemistry: in 2010, Akira Suzuki received the chemistry award for his studies of a carbon–carbon bond-forming reaction that uses boron compounds, together with Richard Heck and Ei-ichi Negishi for their work on other carbon–carbon bond-forming reactions4.
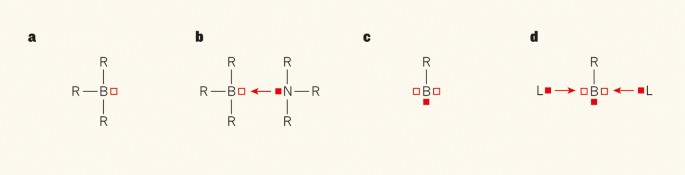
What makes boron compounds so peculiar? Boron atoms have three valence electrons, which can create three bonds by forming pairs with electrons from monovalent groups (R). The resulting borane (BR3) molecules thus have six valence electrons at the boron atom. This is two electrons short of a full valence shell of eight electrons, the number required to give a stable molecule according to the octet rule for main-group atoms. Boranes are therefore Lewis acids, compounds characterized by a 'vacancy' in the valence shell — an absence of a pair of electrons. This vacancy is associated with an empty molecular orbital at the boron atom (Fig. 1a).
a, Borane compounds, BR3, have a 'vacancy' — an absence of a pair of electrons (empty square) — in their shell of valence electrons. R represents any group that can donate a single atom for bond formation. b, Lewis bases, such as tertiary amines (NR3), have a 'lone pair' of electrons (filled square), which they can donate to BR3 to fill the vacancy, forming a complex. c, Borylene compounds have two vacancies and a lone pair. d, Borylene complexes can therefore form when two Lewis-base ligands (L) donate their lone pairs to the borylene. Braunschweig et al.1 report a borylene complex in which the boron atom binds two carbon monoxide ligands.
Lewis acids typically bind strongly to Lewis bases such as amines (NR3), in which the nitrogen atom has a 'lone pair' of electrons in a molecular orbital. The vacancy in the valence shell of Lewis acids can be filled by the lone-pair molecular orbital of Lewis bases, yielding a donor–acceptor bond (Fig. 1b). The resulting compounds are well known to chemists.
In 2012, a theoretical study5 suggested that boron could take part in a different kind of chemical bonding that exhibits an unusual type of donor–acceptor interaction. This involves borylene compounds (BR), in which one boron electron is engaged in a B–R bond and the remaining two electrons form a lone-pair orbital (Fig. 1c). This electronic structure leaves two vacant molecular orbitals at boron. In other words, the borylene is a double Lewis acid (it has two vacancies), but also a Lewis base (one lone-pair molecular orbital). Such borylene species could be stabilized by donor–acceptor interactions with two Lewis bases, forming complexes in which the boron atom retains its lone-pair orbital (Fig. 1d). This bonding situation was proposed for a (BR)L2 molecule6 in which L is a cyclic(alkyl)(amino)carbene (CAAC) ligand — a very strong Lewis base7.
The theoretical study5 predicted that the analogous carbonyl complex (in which L is CO) would possess donor–acceptor bonds that are weaker than those of the CAAC complex, but strong enough to be synthesized. Braunschweig et al. have now verified that prediction by making the borylene dicarbonyl complex (BR)(CO)2, in which R is a bulky substituent. The authors also report unusual geometries and reactivities of related borylene complexes in which L is an isocyanate ligand, CNR.
The researchers observe that the structure and reactivity of (BR)(CO)2 mimics the typical behaviour of transition-metal carbonyl complexes, which is unheard of in the chemistry of main-group elements. For example, when the team irradiated the complex with light, free BR was generated, and it immediately reacted with available substrates. Furthermore, the B–CO bonds in the dicarbonyl complex are significantly shorter than that of a related compound8 in which one of the CO ligands has been replaced by CAAC. The shorter bonds indicate strong π backdonation — a process in which the boron atom's lone pair of electrons is donated to vacant molecular orbitals (known as π* orbitals) of the CO ligands, and which is a typical feature of transition-metal carbonyl complexes.
The isolation of the remarkably stable dicarbonyl complex (BR)(CO)2 also casts light on a controversy about the nature of bonding in compounds in which a main-group atom forms fewer bonds to other atoms than is normal9,10,11 (low-valent compounds). The boron complexes (BR)L2 have the same electronic structure as the carbon compounds CL2. For example, carbon suboxide was reported12 in 1906, and was first thought to be a linear molecule containing sequential double bonds (O=C=C=C=O). But later experiments13 showed that the molecule is bent at 156° at the central carbon atom. It was therefore proposed14 that the compound is a complex of a bare carbon atom with two carbonyl ligands, C(CO)2, and thus exemplifies a previously unrecognized class of compounds called carbones, in which carbon is in the zero oxidation state. Further examples have since been synthesized15,16. The isolation of (BR)(CO)2 shows that related boron compounds are also quite stable, and helps to support the argument that carbones are complexes of bare carbon atoms with ligands.
The synthesis of Braunschweig and colleagues' dicarbonyl complex is just the starting point for further experimental and theoretical work in the field of low-valent boron complexes, and it will build a bridge between the chemistry of main-group compounds and that of transition-metal complexes. Considering the ubiquitous use of transition-metal compounds as catalysts in industrially important reactions, the authors' findings might also open up fresh opportunities in applied chemistry.
Notes
References
Braunschweig, H. et al. Nature 522, 327–330 (2015).
http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1976/
Celik, M. A., Sure, R., Klein, S., Kinjo, R., Bertrand, G. & Frenking, G. Chem. Eur. J. 18, 5676–5692 (2012).
Kinjo, R., Donnadieu, B., Celik, M. A., Frenking, G. & Bertrand, G. Science 333, 610–613 (2011).
Soleilhavoup, M. & Bertrand, G. Acc. Chem. Res. 48, 256–266 (2015).
Dahcheh, F., Martin, D., Stephan, D. W. & Bertrand, G. Angew. Chem. Int. Edn 53, 13159–13163 (2014).
Himmel, D., Krossing, I. & Schnepf, A. Angew. Chem. Int. Edn 53, 370–374 (2014).
Frenking, G. Angew. Chem. Int. Edn 53, 6040–6046 (2014).
Himmel, D., Krossing, I. & Schnepf, A. Angew. Chem. Int. Edn 53, 6047–6048 (2014).
Diels, O. & Wolf, B. Ber. Dt. Chem. Ges. 39, 689–697 (1906).
Jensen, P. & Johns, J. W. C. J. Mol. Spectrosc. 118, 248–266 (1986).
Tonner, R. & Frenking, G. Pure Appl. Chem. 81, 597–611 (2009).
Dyker, C. A., Lavallo, V., Donnadieu, B. & Bertrand, G. Angew. Chem. Int. Edn 47, 3206–3209 (2008).
Fürstner, A., Alcarazo, M., Goddard, R. & Lehmann, C. W. Angew. Chem. Int. Edn 47, 3210–3214 (2008).
Author information
Authors and Affiliations
Corresponding author
Related links
Rights and permissions
About this article
Cite this article
Frenking, G. Peculiar boron startles again. Nature 522, 297–298 (2015). https://doi.org/10.1038/522297a
Published:
Issue Date:
DOI: https://doi.org/10.1038/522297a
This article is cited by
-
Suzuki–Miyaura cross-couplings for alkyl boron reagent: recent developments—a review
Future Journal of Pharmaceutical Sciences (2023)
-
Boron–nitrogen dative bond
Journal of Molecular Modeling (2018)