Abstract
Apoptotic cell death is an essential process in the development of the central nervous system and in the pathogenesis of its degenerative diseases. Efflux of K+ and Cl− ions leads to the shrinkage of the apoptotic cell and facilitates the activation of caspases. Here, we present electrophysiological and immunocytochemical evidences for the activation of a voltage-dependent anion channel (VDAC) in the plasma membrane of neurons undergoing apoptosis. Anti-VDAC antibodies blocked the channel and inhibited the apoptotic process. In nonapoptotic cells, plasma membrane VDAC1 protein can function as a NADH (-ferricyanide) reductase. Opening of VDAC channels in apoptotic cells was associated with an increase in this activity, which was partly blocked by VDAC antibodies. Hence, it appears that there might be a dual role for this protein in the plasma membrane: (1) maintenance of redox homeostasis in normal cells and (2) promotion of anion efflux in apoptotic cells.
Similar content being viewed by others
Introduction
Apoptotic cell death is an essential process in the development of the central nervous system as well as in the pathogenesis of its degenerative diseases.1 An early morphological alteration occurring during apoptosis is cell shrinkage, which is associated with an increased cellular efflux of K+ and Cl− ions.2, 3, 4, 5, 6, 7, 8 The reduced intracellular K+ concentration also facilitates the activation of apoptosis-related proteases – the caspases.9, 10 Conversely, blocking K+ or Cl− channels prevents cell shrinkage and cell death.5, 6, 7, 8 While the K+ efflux depends on an increased number of active K+ channels in the plasma membrane, less is known about the Cl− efflux.
Here, we present electrophysiological evidence for the activation of the voltage-dependent anion channel (VDAC) in the plasma membrane of neuronal cells undergoing apoptosis. VDAC is normally found in the outer mitochondrial membrane, where it is involved in the early stages of certain forms of apoptotic cell death.11 We now report that blocking VDAC activation in the plasma membrane of neural cell lines inhibits the apoptotic process, suggesting a critical role of this channel during the early stages of neuronal apoptosis.
Results and Discussion
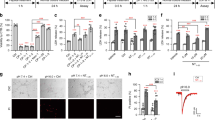
To study electrophysiological changes during apoptosis, we investigated the mouse hippocampal cell line HT22 and the human neuroblastoma cell line SK-N-MC with the patch-clamp technique. Both cell lines displayed low electrical activity under control conditions in isolated membrane patches as well as in whole-cell recordings. The most prominent current was a K+ current of delayed-rectifier type (see below). Cell death was induced by the application of 1 μM staurosporine (STS) for 2 h. Exposed cells exhibited typical apoptotic morphology (cf. Figure 1a with b) with nuclear condensation (Figure 1c) and exposure of phosphatidylserine (PS) on the cell surface (Figure 1d). In addition, at this time point (2 h), there was an apparent release of cytochrome c from the mitochondria (Figure 1e), but yet no caspase activation (data not shown). In accordance with a previous report,5 we found an apoptosis-associated increase in a K+ current of delayed-rectifier type. In whole-cell patch-clamp recordings of HT22 cells, the current was activated by voltage steps more positive than −20 mV, and the K+ channel blocker tetraethylammonium (TEA) at 10 mM reduced the current by 60% (data not shown). Recordings 3–4 min after whole-cell formation showed a dramatic increase in the K+ current for apoptotic cells in comparison to control cells (+180%, P<0.05; Wilcoxon's rank sum test, n=37).
STS induces morphological changes typical for apoptotic cell death. (a and b). Phase-contrast micrographs of control (a) and exposed (b) HT22 cells showing cell shrinkage induced by 1 μM STS for 2 h. (c and d) Apoptotic cells exhibit nuclear condensation as visualized by staining with Hoechst 33358 (arrow in c), and exposure of PS on the outer surface of the plasma membrane, as detected by Annexin V–FITC, a phospholipid-binding protein with high affinity for PS (arrow in d). Scale bar=70 μm in (a and b), and 15 μm in (c and d). (e) Immunoblot showing cytochrome c release from the mitochondria to the cytosol in cells exposed to STS. The quantification of cytosolic cytochrome c was performed by measurement of band density on film after immunoblotting. Values are means±S.E.M. of three determinations. Statistical analysis was performed with the two-tailed Student's t-test (*P<0.001)
VDAC is activated during apoptosis
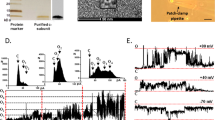
While ion channel currents were relatively infrequent in membrane patches from control cells, a large-conductance ion channel current was frequently seen in apoptotic cells. Hence, in 10% of cell-attached recordings of apoptotic HT22 cells, we observed large square-like single-channel currents (Figure 2a). Excision of the membrane patch from the cell surface increased the activity of the large-conductance channel with a time constant of about 2 min (Figure 2b). The large-conductance channel was found in 48% of the excised inside-out membrane patches from apoptotic cells (24/50), but only occasionally in similar patches from control cells (2/50) (χ2-test P<0.01; Figure 2c). Up to six channels were found in a single-membrane patch. The channel was voltage dependent with an open probability around 70% at 0 mV, which was drastically decreased at both positive and negative voltages (Figure 2d and e). The single-channel conductance was 397 pS, and the reversal potential was 0 mV (Figure 2f). The same large-conductance channel was also seen when apoptosis was induced by 2,3-dimethoxy-1,4-naphthoquinone, methyl mercury, or styrene 7,8-oxide in both HT22 and SK-N-MC cells (data not shown).
Electrophysiological properties of a large-conductance channel in the plasma membrane of (STS-induced) apoptotic HT22 cells. (a) Large-conductance channel in cell-attached mode. Holding voltage (VH, defined as bath voltage−pipette voltage) is 0 mV and test step voltage is −80 mV. Extracellular solution in the pipette and the bath. C and O denotes closed and open states. (b) Consecutive recordings every 20 s. VH=0 mV is followed by 100 ms at +100 mV and 100 ms at −100 mV in each recording. The large-conductance channel is clearly conducting about 2 min after excision of the membrane patch. (c) Frequency diagram of large-conductance channels in cell-attached and -excised inside-out patches for control and (STS-induced) apoptotic cells. (d) Several channels in a patch. Upper current for a voltage-clamp step to +100 mV from VH=0 mV. Lower current for a step to −100 mV. The dashed lines are separated with 37 pA. The figures denote the number of open channels. (e) Open probability after 100 ms at the indicated voltages. VH=0 mV. Data from three consecutive recordings with channels in 10 patches (n=30). (f) I(V) plot for single-channel current in inside-out membrane patches. Conductance is 397±12 pS and the reversal potential is −0.2±2.0 mV (n=6). S.E.M. bars are hidden by the symbols
Thus, there was a much higher channel activity in apoptotic cells compared to control cells in both cell-attached and excised membrane patches (Figure 2c). In addition, this activity increased dramatically when the membrane patch was excised from the intact cell. To investigate the mechanism responsible for this increase in activity, we performed patch-clamp experiments in which the intracellular side of excised inside-out patches was perfused with intracellular solutions with or without either 4 mM ATP, 100 μM cAMP, or 500 μM Ca2+. ATP reversibly blocked the channel activity, while cAMP and Ca2+ had no effect (data not shown). Thus, loss of ATP could possibly contribute to the increase in activity in intact apoptotic cells. To test this hypothesis, we measured ATP levels in HT22 cells exposed to STS for 2 h and found a significant decrease of 38% in cells undergoing apoptosis as compared to control cells. This drop in ATP level is compatible with apoptotic cell death.12
To identify the channel, we characterized its ion selectivity and pharmacological properties. Taken together, our findings are compatible with a VDAC. Changing from an extracellular (mainly NaCl) to an intracellular (mainly KCl) solution in the bath did not affect the current in inside-out membrane patches (data not shown), suggesting that the channel does not select between Na+ and K+ ions. Dilution of the bath solution to 1/5 of its original concentration decreased the current from the (extracellular) pipette solution to the (intracellular) bath solution dramatically, while leaving the current in the opposite direction essentially unchanged (Figure 3a). The reversal potential was shifted from 0 to −41±6 mV (Figure 3b). A cation channel (no Cl− permeability) predicts a shift of +41 mV, a nonselective channel predicts no shift, while a Cl− channel predicts a shift of −41 mV, suggesting that the large-conductance channel is mainly Cl− selective (see equation 1 in Materials and Methods). To further study the ion permeating pore, we exchanged the Cl− ion in the bath solution for the much larger trivalent citrate ion. This reduced the current but did not abolish it (Figure 3c), suggesting that the ion-conducting pore must be relatively wide (>7–8 Å). Taken together, the electrophysiological data ((1) large conductance of about 400 pS, (2) bell-shaped open probability curve, and (3) Cl− selectivity) suggest that the large-conductance channel in the plasma membrane described here is similar to the VDAC, normally present in the mitochondrial outer membrane.13
Selectivity and pharmacological properties of a large-conductance channel in the plasma membrane of (STS-induced) apoptotic HT22 cells. (a) Diluting the bath solution to 1/5 of its original concentration (intracellular side of the patch) reduces the current at −60 mV, but not at +80 mV, VH=0 mV. (b) I(V) plots from control and diluted solutions. Reversal potential is shifted −41 mV, suggesting that the channel is Cl− selective. (c) Citrate3− ions instead of Cl− ions in the bath solution reduces the current at −80 mV, but not at +80 mV
Although there are certain differences between our observations and the reported properties of VDAC,13, 14, 15, 16, 17, 18, 19 this variability may be ascribed to differences in experimental preparations and conditions. For instance, the difference in the (main state) conductance between cellular preparations and VDACs reconstituted in black lipid bilayer depends on different salt concentrations (140 mM vs 1 M). We did not report any small-subconductance state normally seen in VDACs in black lipid bilayer. The reason for this is that these smaller conductances are difficult to separate from other cellular ion channels (e.g. K channels). We also excluded that the activity reported here was due to a volume-sensitive, outwardly rectifying Cl− channel, which has previously been associated with apoptosis.20, 21 This is based on the following observations: (1) The current channel has a much larger conductance in physiological solutions (400 vs 20–80 pS), (2) it has a different voltage dependence of the open probability curve (bell shaped vs sigmoidal), and (3) it has the opposite ATP dependence (decrease vs increase in activity with increasing ATP concentration).
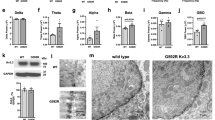
To further establish the identity between the large-conductance channel and VDAC, we performed experiments with two different anti-VDAC antibodies recognizing different epitopes.11 Both antibodies showed similar VDAC-like immunoreactivity in the plasma membrane of HT22 cells (Figure 4a and Figure S1 in Supplementary information). Using the same antibodies on fixed cells preincubated with MitoTracker Red, we observed dot-like cytoplasmatic VDAC immunoreactivity localized in mitochondria (Figure 4b–d). Plasma membrane VDACs were detected in both control and apoptotic cells, suggesting that the channels are constitutively present in the plasma membrane, but activated only during apoptosis.
Immunofluorescence micrographs showing VDAC-like immunoreactivity. (a) Unfixed control HT22 cells incubated with the anti-VDAC antibody Ab2511 displays VDAC-like immunoreactivity in the plasma membrane. (b–d) To visualize mitochondrial VDAC, cells were incubated with MitoTracker Red for 30 min, then fixed with 4% paraformaldehyde and incubated overnight with anti-VDAC antibody Ab25. The micrographs show a control cell stained with VDAC antibody (b) and MitoTracker Red (c). Merging of the images in (b) and (c) demonstrate the mitochondrial localization of VDAC (d). Scale bar=17 μm in (a), and 6 μm in (b–d)
Anti-VDAC antibodies block channel activity and prevent apoptosis
The finding of VDAC-like activity in the plasma membrane of apoptotic cells raises questions about its functional role, and whether it is required for the apoptotic process. We investigated this by occluding the channel with different VDAC blockers: (1) We used three anti-VDAC antibodies (Ab25; Ab31; Ab20) recognizing different epitopes.11 Preincubation of HT22 cells with VDAC antibodies for 30 min prior to exposure to STS blocked plasma membrane VDAC activation (Figure 5a; see also Figure S2a, in Supplementary information) and drastically reduced the number of apoptotic cells, as detected by nuclear condensation (Figure 5b; see also Figure S2b in Supplementary information) or PS exposure (not shown). In contrast, an unrelated antibody (Neurofilament) did not prevent VDAC activity (data not shown). (2) The addition of 240 mM sucrose to the diluted 1/5 solution described above and in Materials and Methods was found to block the single-channel current by 90% (Figure 5c), without affecting the reversal potential. In line with the hypothesis that blocking plasma membrane VDACs also prevents apoptosis, we found a significant decrease in the number of apoptotic cells induced by STS, when they had been preincubated with sucrose (Figure 5d). Most likely, the antiapoptotic effect of sucrose was not influenced by the low Cl− concentration in the medium, which would be expected to increase the Cl− efflux rather than decrease it. Hence, functional VDACs in the plasma membrane are induced by triggers of apoptosis. Blocking these channels prevents apoptosis, suggesting an essential role for plasma membrane VDAC in apoptotic cell death.
Blocking of plasma membrane VDAC prevents apoptosis. (a and b) Preincubation of HT22 cells with different VDAC antibodies (Ab25 or Ab31) for 30 min prior to exposure to STS-blocked plasma membrane VDAC activation (a) and drastically reduced the occurrence of cell death (b). (c) Sucrose (240 mM) added to the diluted (1/5) solution clearly reduce the current at +80 mV. (d) Sucrose also prevented induction of apoptosis by STS as evaluated by the vital triple staining with PI, Hoechst 33358, and Annexin V. Statistical analysis was performed with ANOVA (Fisher's PLSD test). *Significantly different from control or sucrose; #Significantly different from STS (P<0.0001)
Functional expression of plasma membrane VDACs in apoptotic cells seems to occur concomitant with cytochrome c release, but upstream of caspase activation. To ascertain this, we also performed electrophysiological experiments in which cells were exposed to the global caspase inhibitor zVAD-fmk prior to STS. As expected, pretreatment with the caspase inhibitor did not prevent STS-induced VDAC currents (data not shown).
VDAC in the plasma membrane
VDAC was originally detected in the outer mitochondrial membrane, where it mediates the translocation of various metabolites and other compounds in and out of the mitochondria (e.g. pyruvate, malate, ADP, ATP, etc). It has also been found to be an important player in the regulation of mitochondrial cytochrome c release in some forms of apoptosis by interacting with members of the Bcl-2 family of proteins.22, 23 Further, VDAC is one of the components of the mitochondrial permeability transition pore complex, which has also been implicated in the release of cytochrome c leading to caspase activation and apoptosis.24
The presence of VDAC in the plasma membrane has been debated.25 However, several reports, using different techniques, have shown VDAC-like channels in the plasma membrane of multiple cell types, including neurons.14, 15, 16, 26, 27, 28 A major argument against the presence of functional VDACs in the plasma membrane has been that this would result in increased membrane permeability that would not be compatible with cell survival. Therefore, as suggested by Yu and Forte,25 it is likely that these channels are not functional under normal conditions. Our data support this hypothesis, while pointing to a critical role for plasma membrane VDAC in apoptotic cell death.
Recently, it has been demonstrated that the VDAC1 protein in the plasma membrane can function as an NADH (-ferricyanide) reductase previously proposed to be involved in transmembranous redox regulation.27 This finding together with the current demonstration of the appearance of VDAC electrophysiological activity in apoptotic cells suggests a dual role for the plasma membrane VDAC1 protein, that is, maintenance of cellular redox homeostasis in normal cells and cell volume regulation in apoptotic cells. To further investigate this hypothesis, we measured NADH (-ferricyanide) reductase activity in intact control and STS-treated HT22 cells. Cells exposed to 1 μM STS showed a time-dependent increase in NADH (-ferricyanide) reductase activity (Figure 6a). Like the appearance of VDAC electrophysiological activity in apoptotic cells, stimulation of the reductase activity was inhibited by treatment of the cells with anti-VDAC antibodies prior to exposure to STS (Figure 6b). The unrelated antibody Neurofilament did not inhibit the reductace activity. Hence, it appears that both the electrophysiological and NADH (-ferricyanide) reductase activities of the VDAC protein are stimulated in apoptotic cells, and that this stimulation is inhibited by anti-VDAC antibodies.
Stimulation of NADH (-ferricyanide) reducatse activity in apoptotic cells and its partial inhibition by anti-VDAC antibodies. (a) HT22 cells exposed to 1 μM STS showed a time-dependent increase in NADH (-ferricyanide) reductase activity. (b) Preincubation of the cells with anti-VDAC antibodies Ab25 or Ab31 partly prevented the STS-induced increase in NADH (-ferricyanide) reductase activity. Statistical analysis was performed with ANOVA (Fisher's PLSD test). *Significantly different from control or Ab25 or Ab31; #Significantly different from STS (P<0.0001)
In summary, we have shown that the expression of functional VDAC activity is induced by a number of apoptotic stimuli in the plasma membrane of two neuronal cell lines, and that blocking this activity prevents apoptosis. The activation of VDAC appears to be concomitant with mitochondrial cytochrome c release and the opening of K+ channels, but upstream caspase activation. The conjunction of open Cl− and K+ channels leads to Cl− and K+ efflux, cell shrinkage, and further activation of the apoptotic process. In both control and apoptotic cells, the VDAC protein can function as an NADH (-ferricyanide) reductase. How this is regulated, and how the electrophysiological activity of the protein is triggered in apoptotic cells, are subject to current investigation in our laboratories.
Materials and Methods
Cell culture and treatment
Cells (HT22 and SK-N-MC) were incubated in CO2-independent medium (Gibco BRL, Stockholm, Sweden, 18045-054) containing 10% fetal calf serum, 4 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated at 100% relative humidity and at 37°C for 24 h before exposure to the apoptotic stimuli. All chemicals for cell culture were supplied by Life Technologies (Gibco BRL). To induce apoptosis, cells were exposed to 1 μM STS,29 30 μM 2,3-dimethoxy-1,4-naphthoquinone, 4 μM methylmercury, or 0.3 mM styrene 7,8-oxide,30 for 1.5–6.5 h. To prevent apoptosis, cells were preincubated with anti-VDAC antibodies Ab2511 (1 : 200), antibodies Ab2011 (1 : 200), anti-Porin 31 HL Ab-2 (Ab31) (Calbiochem)11 (1 : 100), or the pancaspase inhibitor zVAD-fmk (20 μM). As a negative control, we used an unrelated antibody Neurofilament (DSHB, IA, USA). In some experiments, cells were preincubated with sucrose (240 mM).
Electrophysiology
The electrophysiological recordings were carried out with the patch-clamp technique. We used an EPC-7 patch-clamp amplifier (List Instruments) and pClamp software (Axon Instruments). The extracellular solution was composed of (in mM): 140 NaCl, 5 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, and 23 sucrose (pH 7.4). The intracellular solution was composed of (in mM): 4 NaCl, 140 KCl, 0.5 CaCl2, 1 MgCl2, 10 HEPES, and 5 EGTA (pH 7.4). For the recordings with the citrate ions, instead of using 140 mM NaCl, we used 46.7 mM (Na)3citrate supplemented with 93.3 mM sucrose to retain the osmolarity. The patch pipettes were made of borosilicate glass and the pipette resistance was 4–6 MΩ with the solutions used. In cell-attached and inside-out recordings, the pipettes were filled with the extracellular solution. In the whole-cell recordings, the pipettes were filled with the intracellular solution. In cell-attached and whole-cell recordings, the extracellular solution was used in the bath. In the inside-out recordings presented in the paper, we used the intracellular solution in the bath. We also tried the extracellular solution on the intracellular side of inside-out patches. No difference was seen regarding VDAC activity. The current was always denoted as positive for currents from the intracellular side toward the extracellular pipette side. Leakage current and capacitive currents were removed by subtraction of corresponding traces with no channel activity. For the analysis of the shift of the reversal potential (ΔVrev), we used the following equation:

where R, T, and F have their normal thermodynamic significances, z is the valence of the ion X, and [X] is the concentrations of the ion X in different solutions.
Evaluation of apoptotic cells
The occurrence of apoptosis was evaluated on fixed or living cells. Cells grown on coverslips were fixed with ice-cold methanol/water (8/2=v/v), and stained with cell-impermeable propidium iodide (PI) to visualize nuclear condensation. Apoptotic cells were identified by the smaller size of the nucleus, irregular shape, and brighter intensity of the stained chromatin. For vital stainings, cells grown on coverslips were incubated with a solution of Annexin V–FITC (0.5 μg/ml), which binds to PS, PI (1 μg/ml), and cell-permeable Hoechst 33358 (1 μg/ml) in a buffer containing 10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2. Cells were analyzed with an Olympus BX60 fluorescence microscope equipped with a Hamamatsu digital camera ORCA II.
Immunoblotting and immunocytochemistry
To monitor the release of mitochondrial cytochrome c into the cytosol, the cytosolic fractions from control and exposed cells were separated from the mitochondria.31 Cytochrome c was detected by immunoblotting with a primary mouse antibody (1 : 2500, BD-Pharmingen, San Diego, CA, USA) and with a goat anti-mouse secondary antibody, horseradish peroxidase-conjugated (dilution 1 : 20 000, Pierce Rockford, IL, USA), according to methods described previously.31 Immunoblot bands were quantified with an LKB Ultrascan XL laser densitometer. Immunocytochemistry was performed on unfixed or fixed (4% paraformaldehyde) cells. In order to prevent endocytosis of any added antibodies, living cells were blocked with BSA–PBS for 5 min at 4°C. Fixed or unfixed cells were then incubated overnight at 4°C with two different anti-VDAC antibodies, one raised in rabbit (Ab2511) (1 : 200), and the other one in mouse (anti-Porin 31 HL Ab-2) (1 : 100). After several washes with PBS, fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit or donkey anti-mouse (Jackson) antibodies were added as secondary antibodies for 30 min at 4°C. For control purpose, cells were also incubated with the secondary antibodies alone. In some experiments, living cells were preincubated with the MitoTracker Red (100 nM) (Molecular Probes) for 30 min, fixed and stained as above. Stained cells were analyzed with a fluorescence microscope and images captured as described above, or with a confocal microscope BioRad Radiance Plus.
ATP determination
ATP concentrations were determined in a luminometric assay using the ATP dependency of the light-emitting luciferase-catalyzed oxidation of luciferin (Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's protocol. Briefly, cells (5.0 × 105) were resuspended in 50 μl PBS and 450 μl of boiling lysing buffer (100 mM Tris, 4 mM EDTA (pH 7.75)) were added. Samples were incubated for another 2 min at 100°C and 100 μl were taken out to a 96-well plate. Prior to measurement, 100 μl of luciferase were added to each well and the plate was analyzed in a luminometer (Berthold, R-Biopharm AG, Germany).
NADH (-ferricyanide) reductase activity
Cells (4 × 106) were harvested and incubated in 1 ml buffer, containing 50 mM Tris-HCl (pH 8.0) and 250 μM β-NADH for 5 min at 37°C. The reaction was started by the addition of 250 μM potassium ferricyanide to the reaction buffer leading to reduction of ferricyanide to ferrocyanide. After 10 min, cells were spun down and the concentration of remaining ferricyanide was assessed, using a UNICAM 5625 spectrophotometer, at 420 nm. Ferricyanide reductase activity was calculated as nmol ferricyanide reduced per min per 106 cells.
Abbreviations
- VDAC:
-
voltage-dependent anion channel
- STS:
-
staurosporine
- PS:
-
phosphatidylserine
References
Heidenreich KA (2003) Molecular mechanisms of neuronal cell death. Ann. NY Acad. Sci. 991: 237–250
Barbiero G, Duranti F, Bonelli G, Amenta JS and Baccino FM (1995) Intracellular ionic variations in the apoptotic death of L cells by inhibitors of cell cycle progression. Exp. Cell Res. 217: 408–410
Benson RS, Heer S, Dive C and Watson AJ (1996) Characterization of cell volume loss in CEM-C7A cells during dexamethasone-induced apoptosis. Am. J. Physiol. 270: C1190–C1203
Bortner CD, Hughes Jr FM and Cidlowski JA (1997) A primary role for K+ and Na+ efflux in the activation of apoptosis. J. Biol. Chem. 272: 32436–32442
Yu SP, Yeh C-H, Sensi SL, Gwag BJ, Canzoniero LMT, Farhangrazi ZS, Ying HS, Tian M, Dugan LL and Choi DW (1997) Mediation of neuronal apoptosis by enhancement of outward potassium current. Science 278: 114–117
Colom LV, Diaz ME, Beers DR, Neely A, Xie WJ and Appel SH (1998) Role of potassium channels in amyloid-induced cell death. J. Neurochem. 70: 1925–1934
Wang L, Xu D, Dai W and Lu L (1999) An ultraviolet-activated K+ channel mediates apoptosis of myeloblastic leukemia cells. J. Biol. Chem. 274: 3678–3685
Maeno E, Ishizaki Y, Kanaseki T, Hazama A and Okada Y (2000) Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc. Natl. Acad. Sci. USA 97: 9487–9492
Hughes Jr FM, Bortner CD, Purdy GD and Cidlowski JA (1997) Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J. Biol. Chem. 272: 30567–30576
Cain K, Langlais C, Sun XM, Brown DG and Cohen GM (2001) Physiological concentrations of K+ inhibit cytochrome c-dependent formation of the apoptosome. J. Biol. Chem. 276: 41985–41990
Shimizu S, Matsuoka Y, Shinohara Y, Yoneda Y and Tsujimoto Y (2001) Essential role of voltage dependent anion channel in various forms of apoptosis in mammalian cells. J. Cell Biol. 152: 237–250
Leist M, Single B, Castoldi A, Kuhnle S and Nicotera P (1997) Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J. Exp. Med. 185: 1481–1486
Columbini M, Blachly-Dyson E and Forte M (1996) VDAC, a channel in the outer mitochondrial membrane In Ion Channels, Vol. 4, Narahashi T (ed) (New York: Plenum Press) pp. 169–202
Blatz AL and Magleby KL (1983) Single voltage dependent chloride-selective channels of large conductance in cultured rat muscle. Biophys. J. 43: 237–241
Jalonen T, Johansson S, Holopainen I, Oja SS and Århem P (1989) A high-conductance multi-state anion channel in cultured rat astrocytes. Acta Physiol. Scand. 136: 611–612
Dermietzel R, Hwang TK, Buettner R, Hofer A, Dotzler E, Kremer M, Deutzmann R, Thinnes FP, Fishman GI, Spray DC and Siemen D (1994) Cloning and in situ localization of a brain-derived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proc. Natl. Acad. Sci. USA 91: 499–503
Guibert B, Dermietzel R and Siemen D (1998) Large conductance channel in plasma membranes of astrocytic cells is functionally related to mitochondrial VDAC-channels. Int. J. Biochem. Cell Biol. 30: 379–391
Báthori G, Szabó I, Schmehl I, Tombola F, De Pinto V and Zoratti M (1998) Novel aspects of the electrophysiology of mitochondrial porin. Biochem. Biophys. Res. Comm. 243: 258–263
Bahamonde MI, Fernández-Fernández JM, Guix FX, Vázquez E and Valverde MA (2003) Plasma membrane voltage-dependent anion channel mediates antiestrogen-activated maxi Cl− currents in C1300 neuroblastoma cells. J. Biol. Chem. 278: 33284–33289
Okada Y (1997) Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am. J. Physiol. 273: C755–C789
Shimizu T, Numata T and Okada Y (2004) A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl− channel. Proc. Natl. Acad. Sci. USA 101: 6770–6773
Shimizu S, Narita M and Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399: 483–487
Rostovtseva TK, Antonsson B, Suzuki M, Youle RJ, Colombini M and Bezrukov SM (2004) Bid, but not Bax, regulates VDAC channels. J. Biol. Chem. 279: 13575–13583
Zheng Y, Shi Y, Tian C, Jiang C, Jin H, Chen J, Almasan A, Tang H and Chen Q (2004) Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene 23: 1239–1247
Yu WH and Forte M (1996) Is there VDAC in cell compartments other than the mitochondria? J. Bioenerg. Biomembr. 28: 93–100
Thinnes FP and Reymann S (1997) New findings concerning vertebrate porin. Naturwissenschaften 84: 480–498
Baker MA, Lane DJR, Ly JD, De Pinto V and Lawen A (2004) VDAC1 is a transplasma membrane NADH-ferricyanide reductase. J. Biol. Chem. 279: 4811–4819
Buettner R, Papoutsoglou G, Scemes E, Spray DC and Dermietzel R (2000) Evidence for secretory pathway localization of a voltage-dependent anion channel isoform. Proc. Natl. Acad. Sci. USA 97: 3201–3206
Gorman AM, Hirt UA, Orrenius S and Ceccatelli S (2000) Dexamethasone pre-treatment interferes with apoptotic death in glioma cells. Neuroscience 96: 417–425
Dare E, Tofighi R, Vettori MV, Momoi T, Poli D, Saido TC, Mutti A and Ceccatelli S (2002) Styrene 7,8-oxide induces caspase activation and regular DNA fragmentation in neuronal cells. Brain Res. 933: 12–22
Robertson JD, Enoksson M, Suomela M, Zhivotovsky B and Orrenius S (2002) Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J. Biol. Chem. 277: 29803–29809
Acknowledgements
We thank Peter Larsson (OHSU), Peter Århem (KI), and Roope Männikkö (KI) for comments on this paper; Zhi-Qing David Xu and Vladimir Gogvadze for helping with confocal microscopy and the NADH reductase assay, respectively. This study was supported by grants from the Swedish Research Council (FE, SC), European Commission (CT-2003-506143) (SC), Åke Wibergs Stiftelse (FE), and Magnus Bergvalls Stiftelse (FE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Melino
Supplementary Information accompanies the paper on Cell Death and Diffrentiation website (http://www.nature.com/cdd)
Rights and permissions
About this article
Cite this article
Elinder, F., Akanda, N., Tofighi, R. et al. Opening of plasma membrane voltage-dependent anion channels (VDAC) precedes caspase activation in neuronal apoptosis induced by toxic stimuli. Cell Death Differ 12, 1134–1140 (2005). https://doi.org/10.1038/sj.cdd.4401646
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401646
Keywords
This article is cited by
-
Intracellular amyloid toxicity induces oxytosis/ferroptosis regulated cell death
Cell Death & Disease (2020)
-
Estrogen Signaling in Alzheimer’s Disease: Molecular Insights and Therapeutic Targets for Alzheimer’s Dementia
Molecular Neurobiology (2020)
-
Efficacy of Cannabinoids in a Pre-Clinical Drug-Screening Platform for Alzheimer’s Disease
Molecular Neurobiology (2019)
-
Amyloid proteotoxicity initiates an inflammatory response blocked by cannabinoids
npj Aging and Mechanisms of Disease (2016)
-
The synaptic proteome
Cell and Tissue Research (2015)