Abstract
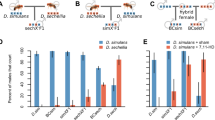
Traits that influence the interactions between males and females can evolve very rapidly through sexual selection1 or sexually antagonistic coevolution2. Rapid change in the fertilization systems of independent populations can give rise to reproductive incompatibilities between populations3,4, and may contribute to speciation5. Here I provide evidence for cryptic reproductive divergence among three sibling species of Drosophila that leads to a form of postmating isolation. When a female mates with both a conspecific and a heterospecific male, the conspecific sperm fertilize the vast majority of the eggs, regardless of the order of the matings. Heterospecific sperm fertilize fewer eggs after these double matings than after single matings. Experiments using spermless males show that the seminal fluid of the conspecific male is largely responsible for this conspecific sperm precedence. Moreover, when two males of the same species mate sequentially with a female from a different species, a highly variable pattern of sperm precedence replaces the second-male sperm precedence that is consistently found within species. These results indicate that females mediate sperm competition, and that second-male sperm precedence is not an automatic consequence of the mechanics of sperm storage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fisher, R. A. The Genetical Theory of Natural Selection (Clarendon, Oxford, 1930).
Rice, W. R. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381, 232–234 (1996).
Palumbi, S. R. & Metz, E. C. Strong reproductive isolation between closely related tropical sea urchins (genus Echinometra). Mol. Biol. Evol. 8, 227–239 (1991).
Lee, Y. H. & Vacquier, V. D. The divergence of species-specific abalone sperm lysins is promoted by positive Darwinian selection. Biol. Bull. 182, 97–104 (1992).
Rice, W. R. & Hostert, E. E. Laboratory experiments on speciation: what have we learned in 40 years? Evolution 47, 1637–1653 (1993).
Parker, G. A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 45, 525–567 (1970).
Pyle, D. W. & Gromko, M. H. Repeated mating by female Drosophila melanogaster: The adaptive importance. Experientia 34, 449–450 (1978).
Chapman, T., Liddle, L. R., Kalb, M. J., Wolfner, M. F. & Partridge, L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373 241–244 (1995).
Hewitt, G. M., Mason, P. & Nichols, R. A. Sperm precedence and homogamy across a hybrid zone in the alpine grasshopper Podisma pedestris. Heredity 62, 343–353 (1989).
Bella, J. L., Butlin, R. K., Ferris, C. & Hewitt, G. M. Asymmetrical homogamy and unequal sex ratio from reciprocal mating-order crosses between Chorthippus parallelus subspecies. Heredity 68, 345–352 (1992).
Gregory, P. G. & Howard, D. J. Apostinsemination barrier to fertilization isolates two closely related ground crickets. Evolution 48, 705–710 (1997).
Wade, M. J., Patterson, H., Chang, N. W. & Johnson, N. A. Postcopulatory, prezygotic isolation in flour beetles. Heredity 72, 163–167 (1994).
Smith, R. L. (ed. Sperm Competition and the Evolution of Animal Mating Systems (Academic, New York, 1984).
Gromko, M. H., Gilbert, D. G. & Richmond, R. C. in Sperm Competition and the Evolution of Animal Mating Systems (ed. Smith, R. L.) 371–426 (Academic, New York, 1984).
Harshman, L. G. & Prout, T. Sperm displacement without sperm transfer in Drosophila melanogaster. Evolution 48, 758–766 (1994).
Clark, A. G., Aguade, M., Prout, T., Harshman, L. G. & Langley, C. H. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics 139, 189–201 (1995).
Hughes, K. A. Quantitative genetics of sperm competition in Drosophila melanogaster. Genetics 145, 139–151 (1997).
David, J., Lemeunier, F., Tsacas, L. & Bocquet, C. Hybridation d'une nouvelle espèce, Drosophila mauritiana avec D. melanogaster et D. simulans. Ann. Génét. 17, 235–241 (1974).
Eberhard, W. G. Female Control: Sexual Selection by Cryptic Female Choice (Princeton Univ. Press, New Jersey, 1996).
Herndon, L. A. & Wolfner, M. F. ADrosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc. Natl Acad. Sci. USA 92, 10114–10118 (1995).
Ingman-Baker, J. & Candido, E. P. Proteins of the Drosophila melanogaster male reproductive system: two-dimensional gel patterns of proteins synthesized in the XO, XY, and XYY testis and paragonial gland and evidence that the Y chromosome does not code for structural sperm proteins. Biochem. Genet. 18, 809–828 (1980).
Coyne, J. A. The genetics of an isolating mechanism between two sibling species of Drosophila. Evolution 47, 778–788 (1993).
Acknowledgements
I thank K. C. Price, P. Rooney, K. Kyle, C. Kim and K. Dyer for technical assistance; J. Coyne for inspiration and help; and H. A. Orr, M. Turelli, M. Noor, N. Johnson and M. Wade for comments. This work was supported by an NSF predoctoral fellowship to the author, and by an NIH grant to J. Coyne.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Price, C. Conspecific sperm precedence in Drosophila. Nature 388, 663–666 (1997). https://doi.org/10.1038/41753
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/41753
This article is cited by
-
Reproductive interference and Satyrisation: mechanisms, outcomes and potential use for insect control
Journal of Pest Science (2022)
-
The resilience of reproductive interference
Evolutionary Ecology (2021)
-
Reproductive Isolation through Experimental Manipulation of Sexually Antagonistic Coevolution in Drosophila melanogaster
Scientific Reports (2017)
-
Asymmetrical sexual isolation but no postmating isolation between the closely related species Drosophila suboccidentalis and Drosophila occidentalis
BMC Evolutionary Biology (2015)
-
Using RNA sequencing to characterize female reproductive genes between Z and E Strains of European Corn Borer moth (Ostrinia nubilalis)
BMC Genomics (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.