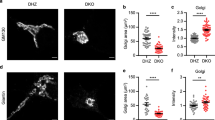
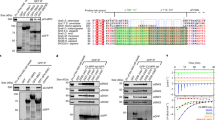
Abstract
In eukaryotic cells, the Golgi apparatus receives newly synthesized proteins from the endoplasmic reticulum (ER) and delivers them after covalent modification to their destination in the cell. These proteins move from the inside (cis) face to the plasma-membrane side (trans) of the Golgi, through a stack of cisternae, towards the trans-Golgi network (TGN), but very little is known about how proteins are moved through the Golgi compartments. In a model known as the maturation model1,2,3, no special transport process was considered necessary, with protein movement along the Golgi being achieved by maturation of the cisternae. Alternatively, proteins could be transported by vesicles4,5,6 or membrane tubules7,8. Although little is known about membrane-tubule-mediated transport7,8, the molecular mechanism for vesicle-mediated transport is quite well understood, occurring through docking of SNAREs on the vesicle with those on the target membrane4,5,6,7,8,9,10,11,12,13. We have now identified a protein of relative molecular mass 27K which is associated with the Golgi apparatus. The cytoplasmic domain of this protein or antibodies raised against it quantitatively inhibit transport in vitro from the ER to the trans-Golgi/TGN, acting at a stage between the cis/medial- and the trans-Golgi/TGN. This protein, which behaves like a SNARE and has been named GS27 (for Golgi SNARE of 27K), is identical to membrin, a protein implicated earlier in ER-to-Golgi transport14. Our results suggest that protein movement from medial- to the trans-Golgi/TGN depends on SNARE-mediated vesicular transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lippincott-Schwartz, J. Bidirectional membrane traffic between the endoplasmic reticulum and Golgi apparatus. Trends Cell Biol. 3, 81–88 (1993).
Saraste, J. & Svensson, K. Pathways of protein sorting and membrane traffic between the rough endoplasmic reticulum and the Golgi complex. Semin. Cell Biol. 3, 343–355 (1992).
Bannykh, S. I. & Balch, W. E. Membrane dynamics at the endoplasmic reticulum–Golgi interface. J. Cell Biol. 138, 1–4 (1997).
Palade, G. E. Intercellular aspects of the processing of protein synthesis. Science 189, 347–354 (1975).
Rothman, J. E. & Wieland, F. T. Protein sorting by transport vesicles. Science 272, 227–234 (1996).
Schekman, R. & Orci, L. Coat proteins and vesicle budding. Science 271, 1526–1532 (1996).
Klausner, R. D., Donaldson, J. G. & Lippincott-SSchwartz, J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116, 1071–1080 (1992).
Mellman, I. & Simons, K. The Golgi complex: in vitro veritas? Cell 68, 829–840 (1992).
Hong, W. Protein Trafficking along the Exocytotic Pathway(Landes, Austin, USA, (1996)).
Rothman, J. E. Mechanism of intracellular protein transport. Nature 372, 55–63 (1994).
Söllner, T. et al. SNAP receptors implicated in vesicle targeting and fusion. Nature 362, 318–324 (1993).
Whiteheart, S. W. & Kubalek, E. W. SNAPs and NSF: general members of the fusion apparatus. Trends Cell Biol. 5, 64–69 (1995).
Pfeffer, S. R. Transport vesicle docking: SNAREs and associates. Annu. Rev. Cell Dev. Biol. 12, 441–461 (1996).
Hay, J. C., Chao, D. S., Kuo, C. S. & Scheller, R. H. Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell 89, 149–158 (1997).
Lian, J. P. & Ferro-Novickk, S. Bos1p, an integral membrane protein of the endoplasmic reticulum to Golgi transport vesicles, is required for their fusion competence. Cell 73, 735–745 (1993).
Newman, A. P., Groesch, M. E. & Ferro-Novickk, S. Bos1p, a membrane protein required for ER to Golgi transport in yeast, co-purifies with carrier vesicles and with Bet1p and the ER membrane. EMBO J. 11, 3609–3617 (1992).
Shim, J., Newman, A. P. & Ferro-Novickk, S. The BOS1 gene encodes an essential 27-kD putative membrane protein that is required for vesicular transport from the ER to the Golgi complex in yeast. J. Cell Biol. 113, 55–64 (1991).
Lowe, S. L., Wong, S. H. & Hong, W. The mammalian ARF-like protein 1 (Ar11) is associated with the Golgi complex. J. Cell Sci. 109, 209–220 (1996).
Balch, W. E., McCaffery, J. M., Plutner, H. & Farquhar, M. G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell 76, 841–852 (1994).
Beckers, C. J. M., Keller, D. S. & Balch, W. E. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell 50, 523–534 (1987).
Davidson, H. W. & Balch, W. E. Differential inhibition of multiple vesicular transport steps between the endoplasmic reticulum and trans Golgi network. J. Biol. Chem. 268, 4216–4226 (1993).
Nagahama, M. et al. Av-SNARE implicated in intra-Golgi transport. J. Cell Biol. 133, 507–516 (1996).
Subramaniam, V. N., Peter, F., Philp, R., Wong, S. H. & Hong, W. GS28, a 28-kilodalton Golgi SNARE that participates in ER–Golgi transport. Science 272, 1161–1163 (1996).
Plutner, H., Davidson, H. W., Saraste, J. & Balch, W. R. Morphological analysis of protein transport from the endoplasmic reticulum to Golgi membranes in digitonin permeabilized cells: role of the p58 containing compartment. J. Cell Biol. 119, 1077–1096 (1992).
Wilson, D. W., Whiteheart, S. W., Wiedmann, M., Brunner, M. & Rothman, J. E. Amultisubunit particle implicated in membrane fusion. J. Cell Biol. 117, 531–538 (1992).
Søgaard, M. et al. Arab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell 78, 937–948 (1994).
McNew, J. A. et al. Ypt6p, a prenylated SNARE essential for endoplasmic reticulum–Golgi transport. J. Biol. Chem. 272, 17776–17783 (1997).
Guan, K. & Dixon, J. E. Eukaryotic proteins expressed in Escherichia coli: An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Analyt. Biochem. 192, 262–267 (1991).
Acknowledgements
We thank J. E. Rothman for his generous gift of plasmids for production of recombinant His ? 6-NSF and His ? 6-α-SNAP, members of W.H.'s laboratory for critically reading the manuscript and Y. H. Tan for his continuous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lowe, S., Peter, F., Subramaniam, V. et al. A SNARE involved in protein transport through the Golgi apparatus. Nature 389, 881–884 (1997). https://doi.org/10.1038/39923
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/39923
This article is cited by
-
A draft map of the mouse pluripotent stem cell spatial proteome
Nature Communications (2016)
-
GOSR2 Lys67Arg Is Associated With Hypertension in Whites
American Journal of Hypertension (2009)
-
PRA1 promotes the intracellular trafficking and NF-κB signaling of EBV latent membrane protein 1
The EMBO Journal (2006)
-
Membrane localization of the U2 domain of Protein 4.1B is necessary and sufficient for meningioma growth suppression
Oncogene (2005)
-
COPI-mediated protein and lipid sorting in the early secretory pathway
Protoplasma (1999)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.