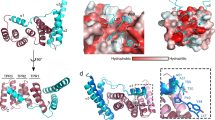
Abstract
Many bacterial pathogens use a type III protein secretion system to deliver virulence effector proteins directly into the host cell cytosol, where they modulate cellular processes1,2. A requirement for the effective translocation of several such effector proteins is the binding of specific cytosolic chaperones, which typically interact with discrete domains in the virulence factors3,4,5. We report here the crystal structure at 1.9 Å resolution of the chaperone-binding domain of the Salmonella effector protein SptP with its cognate chaperone SicP. The structure reveals that this domain is maintained in an extended, unfolded conformation that is wound around three successive chaperone molecules. Short segments from two different SptP molecules are juxtaposed by the chaperones, where they dimerize across a hydrophobic interface. These results imply that the chaperones associated with the type III secretion system maintain their substrates in a secretion-competent state that is capable of engaging the secretion machinery to travel through the type III apparatus in an unfolded or partially folded manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Galán, J. E. & Collmer, A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284, 322–328 (1999).
Cornelis, G. R. & Van Gijsegem, F. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54, 735–774 (2000).
Wattiau, P., Bernier, B., Deslée, P., Michiels, T. & Cornelis, G. R. Individual chaperones required for Yop secretion by Yersinia. Proc. Natl Acad. Sci. USA 91, 10493–10497 (1994).
Wattiau, P., Woestyn, S. & Cornelis, G. R. Customized secretion chaperones in pathogenic bacteria. Mol. Microbiol. 20, 255–262 (1996).
Bennett, J. C. Q. & Hughes, C. From flagellum assembly to virulence: the extended family of type III export chaperones. Trends Microbiol. 8, 202–204 (2000).
Kaniga, K., Uralil, J., Bliska, J. B. & Galán, J. E. A secreted tyrosine phosphatase with modular effector domains encoded by the bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 21, 633–641 (1996).
Fu, Y. & Galán, J. E. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401, 293–297 (1999).
Stebbins, C. E. & Galan, J. E. Modulation of host signaling by a bacterial mimic. Structure of the Salmonella effector SptP bound to Rac1. Mol. Cell 6, 1449–1460 (2000).
Galán, J. E. & Zhou, D. Striking a balance: modulation of the actin cytoskeleton by Salmonella. Proc. Natl Acad. Sci. USA 97, 8754–8761 (2000).
Fu, Y. & Galán, J. E. Identification of a specific chaperone for SptP, a substrate of the centisome 63 type III secretion system of Salmonella typhimurium. J. Bacteriol. 180, 3393–3399 (1998).
Evdokimov, A. G., Tropea, J. E., Routzahn, K. M., Copeland, T. D. & Waugh, D. S. Structure of the N-terminal domain of Yersinia pestis YopH at 2.0 Å resolution. Acta Crystallogr. D 57, 793–799 (2001).
Smith, C. L., Khandelwal, P., Keliikuli, K., Zuiderweg, E. R. P. & Saper, M. A. Structure of the type III secretion and substrate-binding domain of Yersinia YopH phosphatase. Mol. Microbiol. (in the press).
Kubori, T. et al. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280, 602–605 (1998).
Blocker, A. et al. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147, 683–693 (1999).
Stebbins, C. E., Kaelin, W. G. Jr & Pavletich, N. P. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science 284, 455–461 (1999).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307 (1997).
Terwilliger, T. C. & Berendzen, J. Automated structure solution for MIR and MAD. Acta Crystallogr. D 55, 849–861 (1999).
Terwilliger, T. C. Maximum likelihood density modification. Acta Crystallogr. D 56, 965–972 (2000).
Perrakis, A., Morris, R. M. & Lamzin, V. S. Automated protein model building combined with iterative structure refinement. Nature Struct. Biol. 6, 458–463 (1999).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Kraulis, P. J. Molscript: a program to produce both detailed and schematic plits or protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Merrit, E. A. & Murphy, M. E. Raster3D Version 2.0: a program for photorealistic molecular graphics. Acata Crystallogr. D 50, 869–873 (1994).
Guex, N. & Peitsch, M. C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Acknowledgements
We thank the following people for access to and assistance with crystallographic equipment: at Yale University School of Medicine, P. Pepin of the Macromolecular X-ray Crystallography Facility, and S. Kunchaparty and A.-M. Quinn of the Research Computing Group for workstation access; at the CHESS, D. Szebenyi and the MacChess staff; B. Sweet, A. Saxena and the staff of Brookhaven beamline X12C. We also acknowledge N. Papavasiliou, P. Jeffrey, S. Fugmann and members of the Galán laboratory for discussions and critical reading of this manuscript before submission. C.E.S. was supported by a fellowship of the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation. This work was funded by Public Health Services grants to J.E.G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stebbins, C., Galán, J. Maintenance of an unfolded polypeptide by a cognate chaperone in bacterial type III secretion. Nature 414, 77–81 (2001). https://doi.org/10.1038/35102073
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35102073
This article is cited by
-
Substrate-engaged type III secretion system structures reveal gating mechanism for unfolded protein translocation
Nature Communications (2021)
-
Arginine glycosylation enhances methylglyoxal detoxification
Scientific Reports (2021)
-
Xanthomonas effector XopR hijacks host actin cytoskeleton via complex coacervation
Nature Communications (2021)
-
Salmonella-based platform for efficient delivery of functional binding proteins to the cytosol
Communications Biology (2020)
-
Type three secretion system in Salmonella Typhimurium: the key to infection
Genes & Genomics (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.