Abstract
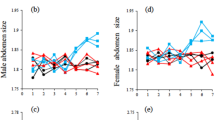
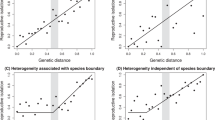
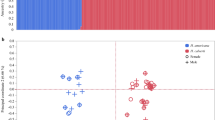
Sexual selection may facilitate speciation because it can cause rapid evolutionary diversification of male mating signals and female preferences. Divergence in these traits can then contribute to reproductive isolation1,2,3. The sensory drive hypothesis predicts that three mechanisms underlie divergence in sexually selected traits4: (1) habitat-specific transmission of male signals5,6,7; (2) adaptation of female perceptual sensitivity to local ecological conditions8; and (3) matching of male signals to female perceptual sensitivity4,9. I test these mechanisms in threespine sticklebacks (Gasterosteus spp.) that live in different light environments. Here I show that female perceptual sensitivity to red light varies with the extent of redshift in the light environment, and contributes to divergent preferences. Male nuptial colour varies with environment and is tuned to female perceptual sensitivity. The extent of divergence among populations in both male signal colour and female preference for red is correlated with the extent of reproductive isolation in these recently diverged species. These results demonstrate that divergent sexual selection generated by sensory drive contributes to speciation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fisher, R. A. The Genetical Theory of Natural Selection (Clarendon, Oxford, 1930).
Lande, R. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725 (1981).
West-Eberhard, M. J. Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 (1983).
Endler, J. A. Signals, signal conditions, and the direction of evolution. Am. Nat. 139, 125–153 (1992).
Ryan, M. J. & Wilczynski, W. Evolution of intraspecific variation in the advertisement call of a cricket frog (Acris crepitans, Hylidae). Biol. J. Linn. Soc. 44, 249–271 (1991).
Marchetti, K. Dark habitats and bright birds illustrate the role of the environment in species divergence. Nature 362, 149–152 (1993).
Seehausen, O., van Alphen, J. J. M. & Witte, F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277, 1808–1811 (1997).
Levine, J. S. & MacNichol, E. F. Jr Visual pigments in teleost fishes: effects of habitat, microhabitat, and behavior on visual system evolution. Sensory Process. 3, 95–131 (1979).
Ryan, M. Sexual selection, sensory systems, and sensory exploitation. Oxf. Surv. Evol. Biol. 7, 156–195 (1990).
McFarland, W. N. & Munz, F. W. Part III: The evolution of photopic visual pigments in fishes. Vision Res. 12, 1071–1080 (1975).
Reimchen, T. E. Loss of nuptial color in threespine sticklebacks (Gasterosteus aculeatus). Evolution 43, 450–460 (1989).
Lythgoe, J. N. The Ecology of Vision (Oxford Univ. Press, 1979).
Endler, J. A. Variation in the appearance of guppy color patterns to guppies and their predators under different visual conditions. Vision Res. 31, 587–608 (1991).
McPhail, J. D. Ecology and evolution of sympatric sticklebacks (Gasterosteus): morphological and genetic evidence for a species pair in Enos Lake, British Columbia. Can. J. Zool. 62, 1402–1408 (1984).
McPhail, J. D. Ecology and evolution of sympatric sticklebacks (Gasterosteus): evidence for a species pair in Paxton Lake, Texada Island, British Columbia. Can. J. Zool. 70, 361–369 (1992).
McPhail, J. D. in The Evolutionary Biology of the Threespine Stickleback (eds Bell, M. A. & Foster, S. A.) 399–437 (Oxford Univ. Press, 1994).
Schluter, D. in Endless Forms: Species and Speciation (eds Howard, D. J. & Berlocher, S. H.) 114–129 (Oxford Univ. Press, 1998).
Cronly-Dillon, J. & Sharma, S. C. Effect of season and sex on the photopic spectral sensitivity of the three-spined stickleback J. Exp. Biol. 49, 679–687 (1968).
Martins, E. P. & Hansen, T. F. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 149, 646–667 (1997).
Nagel, L. & Schluter, D. Body size, natural selection, and speciation in sticklebacks. Evolution 52, 209–218 (1998).
Kodric-Brown, A. & Brown, J. H. Truth in advertising: the kinds of traits favored by sexual selection. Am. Nat. 124, 309–323 (1984).
Schluter, D. & Price, T. D. Honesty, perception, and population divergence in sexually selected traits. Proc. R. Soc. Lond. B 253, 117–122 (1993).
Lande, R. & Kirkpatrick, M. Ecological speciation by sexual selection. J. Theor. Biol. 133, 85–98 (1988).
Higashi, M., Takimoto, G. & Yamamura, N. Sympatric speciation by sexual selection. Nature 402, 523–526 (1999).
Schluter, D. Experimental evidence that competition promotes divergence in adaptive radiation. Science 266, 798–801 (1994).
Podos, J. Correlated evolution of morphology and vocal signal structure in Darwin's finches. Nature 409, 185–188 (2001).
McDonald, C. G. & Hawryshyn, C. W. Intraspecific variation of spectral sensitivity in threespine stickleback (Gasterosteus aculeatus) from different photic regimes. J. Comp. Physiol. A 176, 255–260 (1995).
Klump, G. M., Dooling, R. J., Fay, R. R. & Stebbins, W. C. Methods in Comparative Psychoacoustics (Birkhauser, Basel, Switzerland, 1995).
Rundle, H. D., Nagel, L. M., Boughman, J. W. & Schluter, D. Natural selection and parallel speciation in sympatric sticklebacks. Science 287, 306–308 (2000).
Taylor, E. B. & McPhail, J. D. Historical contingency and ecological determinism interact to prime speciation in sticklebacks, Gasterosteus. Proc. R. Soc. Lond. B 267, 2375–2384 (2000).
Acknowledgements
I would like to thank S. Morgan and B. Harvey for help with data collection, and C. Hawryshyn (and his NSERC equipment grant) and P. Jolliffe for the loan of equipment. I also thank J. Endler, S. Kuhnholz, T. Lenormand, S. Otto, A. Poon, H. Rundle, G. Saxer, D. Schluter, J. Smith, S. Vamosi and M. Whitlock for comments on the manuscript; Holnam West Materials for access to Paxton Lake; and Fairwinds Corporation for access to Enos Lake. This work was supported by NSF-NATO and NSF-International Research fellowships to J.W.B. and NSERC operating grants to D. Schluter.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boughman, J. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411, 944–948 (2001). https://doi.org/10.1038/35082064
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35082064
This article is cited by
-
Divergent preference functions generate directional selection in a jumping spider
Scientific Reports (2023)
-
Geographic Drivers of Genetic and Plumage Color Diversity in the Blue-Crowned Manakin
Evolutionary Biology (2023)
-
Neurogenomic divergence during speciation by reinforcement of mating behaviors in chorus frogs (Pseudacris)
BMC Genomics (2021)
-
Multilocus phylogeography and ecological niche modeling suggest speciation with gene flow between the two Bamboo Partridges
Avian Research (2021)
-
Chemical signal divergence among populations influences behavioral discrimination in the whiptail lizard Aspidoscelis lineattissimus (squamata: teiidae)
Behavioral Ecology and Sociobiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.