Abstract
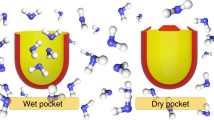
Many biomolecules are characterized by surfaces containing extended nonpolar regions1, and the aggregation and subsequent removal of such surfaces from water is believed to play a critical role in the biomolecular assembly in cells2. A better understanding of the hydrophobic hydration of biomolecules may therefore yield new insights into intracellular assembly. Conventional views hold that the hydration shell of small hydrophobic solutes is clathrate-like, characterized by local cage-like hydrogen-bonding structures and a distinct loss in entropy2. The hydration of extended nonpolar planar surfaces, however, appears to involve structures that are orientationally inverted relative to clathrate-like hydration shells3,4, with unsatisfied hydrogen bonds that are directed towards the hydrophobic surface. Here we present computer simulations of the interaction between the polypeptide melittin and water that demonstrate that the two different hydration structures also exist near a biomolecular surface. We find that the two structures are distinguished by a substantial difference in the water–water interaction enthalpy, and that their relative contributions depend strongly on the surface topography of the melittin molecule: clathrate-like structures dominate near convex surface patches, whereas the hydration shell near flat surfaces fluctuates between clathrate-like and less-ordered or inverted structures. The strong influence of surface topography on the structure and free energy of hydrophobic hydration is likely to hold in general, and will be particularly important for the many biomolecules whose surfaces contain convex patches, deep or shallow concave grooves and roughly planar areas5.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Teeter, M. M. Water–protein interactions: Theory and experiment. Annu. Rev. Biophys. Biophys. Chem. 20, 577–600 (1991).
Blokzijl, W. & Engberts, J. B. F. N. Hydrophobic effects. Opinions and facts. Angew. Chem. Int. Edn Engl. 32, 1545–1579 (1993).
Lee, C. Y., MCammon, J. A. & Rossky, P. J. The structure of liquid water at an extended hydrophobic surface. J. Chem. Phys. 80, 4448–4455 (1984).
Du, Q., Freysz, E. & Shen, Y. R. Surface vibrational spectroscopic studies of hydrogen bonding and hydrophobicity. Science 264, 826–828 (1994).
Kuhn, L. A. et al. The interdependence of protein surface topography and bound water molecules revealed by surface accessibility and fractal density measures. J. Mol. Biol. 228, 13–22 (1992).
Sharp, K. A., Nicholls, A., Fine, R. F. & Honig, B. Reconciling the magnitude of the microscopic and macroscopic hydrophobic effects. Science 252, 106–109 (1991).
Sanchez-Ruiz, J. M. Molecular-size corrections to the strength of the hydrophobic effect: A critical review. Eur. Biophys. J. 24, 261–274 (1996).
Dempsey, C. E. The actions of melittin on membranes. Biochim. Biophys. Acta 1031, 143–161 (1990).
Terwilliger, T. C. & Eisenberg, D. The structure of melittin. I. Structure determinations and partial refinement. J. Biol. Chem. 257, 6010–6015 (1982).
Clarke, A. R. & Lund, P. A. in The ChaperoninsCh. 7 (ed. Ellis, R. J.) 167–212 (Academic, San Diego, 1996).
Bernstein, F. C. et al. The protein data bank: A computer-based archival file for macromolecular structures. J. Biol. Chem. 112, 535–542 (1977).
Kitao, A., Hirata, F. & Go, N. The effects of solvent on the conformation and collective motions of protein: Normal mode analysis and molecular dynamics simulations of melittin in water and in vacuum. Chem. Phys. 158, 447–472 (1991).
Mehrotra, P. K. & Beveridge, D. L. Structural analysis of molecular solutions based on quasi-component distribution functions. Application to [H2CO]aq at 25 °C. J. Am. Chem. Soc. 102, 4287–4294 (1980).
Zichi, D. A. & Rossky, P. J. The equilibrium solvation structure for the solvent-separated hydrophobic bond. J. Chem. Phys. 83, 797–808 (1985).
Rossky, P. J. & Karplus, M. Solvation. A molecular dynamics study of a dipeptide in water. J. Am. Chem. Soc. 101, 1913–1937 (1979).
Pascual-Ahuir, J. L., Silla, E. & Tunon, I. GEPOL: An improved description of molecular surfaces. III. A new algorithm for the computation of the solvent-excluding surface. J. Comput. Chem. 15, 1127–1138 (1994).
Freire, E., Murphy, K. P., Sanchez-Ruiz, J. M., Galiseo, M. L. & Privalov, P. L. The molecular basis of cooperativity in protein folding. Thermodynamic dissection of interdomain interactions in phosphoglycerate kinase. Biochemistry 31, 250–256 (1992).
Berendsen, H. J. C., Postma, J. P. M., van Gunsteren, W. F. & Hermans, J. in Intermolecular Forces (ed. Pullman, B.) 331–342 (Reidel, Dordrecht, 1981).
Jorgensen, W. L. & Tirado-Rives, J. The OPLS potential functions for proteins. Energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 110, 1657–1671 (1988).
Verlet, L. Computer “experiments” on classical fluids. I. Thermodynamical properties of Lennard–Jones molecules. Phys. Rev. 159, 98–103 (1967).
Ryckaert, J.-P., Ciccotti, G. & Berendsen, H. J. C. Numerical integration of the Cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 23, 327–341 (1977).
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: Insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991).
Acknowledgements
We acknowledge contributions from W. S. Sheu and T. S. Cohen in the early stages of this work. This work was supported by the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, YK., Rossky, P. Surface topography dependence of biomolecular hydrophobic hydration. Nature 392, 696–699 (1998). https://doi.org/10.1038/33653
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/33653
This article is cited by
-
Scrutinizing the protein hydration shell from molecular dynamics simulations against consensus small-angle scattering data
Communications Chemistry (2023)
-
Hydrophobic Hydration: A Theoretical Investigation of Structure and Dynamics
Journal of Chemical Sciences (2023)
-
Sticky when dry
Nature Chemistry (2020)
-
Biophysical Spandrels form a Hot-Spot for Kosmotropic Mutations in Bacteriophage Thermal Adaptation
Journal of Molecular Evolution (2019)
-
Real-time monitoring of hydrophobic aggregation reveals a critical role of cooperativity in hydrophobic effect
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.