Abstract
Study design:
Prospective cohort study with medical record review.
Objective:
To evaluate the clinical utility of an infection control program in a patient cohort at high risk for methicillin-resistant Staphylococcus aureus (MRSA) infection and to identify risk factors interfering with successful decolonization of MRSA.
Setting:
All spinal cord injured (SCI) patients hospitalized at the Swiss Paraplegic Center (SPC) Nottwil from April 1991 to April 2001.
Methods:
Patients whose medical records indicated laboratory-confirmed MRSA colonization or infection were included. Incidence of MRSA colonization or infection was classified as community acquired, nosocomial or transferred based on standardized criteria. Risk factors for community-acquired MRSA colonization in SCI patients were determined. MRSA subtyping and identification of nosocomial spread was performed through pulse-field gel electrophoresis (PFGE).
Results:
Of 5992 admissions, 100 episodes of MRSA (colonization 22 cases, infection 78 cases) were identified among 76 patients. Overall incidence (1991–2001) per 1000 patient days was 0.26 cases on admission compared to 0.08 at discharge (P<0.001). Community-acquired MRSA was most frequent (56%) followed by nosocomial acquisition (34%). PFGE subtyping identified two nosocomial clusters with six and three cases, respectively. Most of community-acquired MRSA isolates were genetically unrelated and also distinct from epidemic strains identified in Switzerland during the study period. Decolonization was successful in 60 of 76 (78.9%) MRSA-positive patients.
Conclusion:
In the largest European SCI center, MRSA controlling is feasible if infection control policies are vigorously applied.
Similar content being viewed by others
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) has become the most important emerging pathogen in medicine,1 but only few data are published in the particular setting of spinal cord injury (SCI) where intensive care units and long-term hospitalization wards are parts of a holistic rehabilitation program.2 Thus, Mylotte et al.,3 in an 18 months short-term prospective study including 63 admissions, identified MRSA as the most common multi-resistant organism colonizing SCI patients. However, a detailed long-term prospective epidemiologic MRSA study in rehabilitation of SCI patients has, as to our knowledge, not yet been published. Such data might be of major epidemiological interest since the recent advantages in molecular typing of MRSA revealed that only a few genotypically distinct strains were at the origin of the worldwide dissemination of MRSA.4 Thus, control measures focusing on prevention of transmission appear to be primordial and the emergence of vancomycin-resistant MRSA promotes prevention even more than in the past.5 We prospectively examined over a 10-year period the impact of a prevention strategy on endemic and epidemic MRSA to evaluate the clinical utility of such a program.
Patients and methods
Study design
In a prospective study from April 1991 to April 2001, we collected data of all episodes of MRSA colonization or infection of in-patients at the Swiss Paraplegic Center (SPC) Nottwil, Switzerland. For each MRSA case, detailed epidemiological and clinical data were collected. Severity and impact of associated diseases were classified according to the Charlson's weighted index of comorbidity.6 SCI was classified according to the American Spinal Injury Association (ASIA) impairment scale.7
Definitions
Infections were defined according to the Centers for Disease Control and Prevention (CDC) criteria.8 In patients at risk (from southern and south-western Switzerland, from abroad, transfers from intensive care units, earlier MRSA carrier), extended admission screening according to standard operating procedures routinely included swabs from nose, axilla, groin, urinary tract (if a permanent catheter was present) and wounds. Additional cultures were performed on clinical evidence. Overall 1622 patients (27.1% of admissions) fulfilled the criteria for patients at risk. Patients were considered as MRSA carriers, when at least one culture of any swabbed body site showed MRSA. They were classified as MRSA-negative when three swab series over a period of 14 days remained negative (including nose, axilla, groin and all once positive sites) and without antibiotic treatment with activity against MRSA. The mode of MRSA acquisition—nosocomial versus non-nosocomial—was classified by CDC criteria, including the results of pulsed-field gel electrophoresis (PFGE) pattern of the isolated strains classified according to Tenover et al.9, 10 Community acquisition was assumed, if extended screening for MRSA was positive within 48 h from admission, and no hospitalization was recorded in the previous year. If extended screening at admission was negative, proved nosocomial acquisition was considered; if no extended screening occurred suspected nosocomial acquisition was assumed. Nosocomial clusters were defined as two or more patients who were hospitalized in the same ward within a 6-month period and yielding related MRSA strains.11 Transferred cases were defined as (1) MRSA identification beyond 48 h after transfer from another institution, (2) absence of any epidemiological link, and (3) isolation of a new, previously unknown molecular subtype. According to Tenover et al.,10 bacterial isolates were determined as genetically related, if PFGE pattern differed in maximum six restriction fragments; isolates with a difference of more than six restriction fragments were considered as unrelated (different types).10 Genetically related strains were classified in groups A to K, unrelated strains subsumed in group X, untyped strains in group O.
Infection control measures
They have been established in 1991; the infection control team remained basically unchanged for 10 years. Contact isolation of patients admitted (1) from health-care institutions from abroad or from southern and south-western regions of Switzerland, (2) from a burn or intensive care unit, (3) with known previous MRSA positivity was performed according to the CDC guidelines,12 until results from screening were available. Patients yielding MRSA during hospitalization were also placed in strict contact isolation. In primarily isolated patients, standardized screening for MRSA was performed within 48 h from admission. Isolation of MRSA-positive individuals was maintained until three consecutive swab series over a period of 14 days remained negative, including screening at discharge. Health-care workers employed in wards with nosocomial MRSA-positive patients were routinely screened.
Microbiologic evaluation
Standardized MRSA screening included swabs from the following body sites: nose, axilla, groin, urine (in case of permanent catheter) and decubital ulcers or other skin lesions. Susceptibility-testing was performed and interpreted according to the guidelines of the National Committee for Clinical Laboratory Standards (NCCLS)13 using disc diffusion technology (Kirby–Bauer). Molecular typing of MRSA isolates was performed using PFGE. After digestion of the genomic DNA by SmaI (New England Biolabs, Basel, Switzerland), the restriction fragments were separated by PFGE using a temperature-controlled CHEF DR III system (Bio-Rad Laboratories, Reinach, Switzerland), as previously described.14 For PFGE pattern analysis, the software GelCompar (Applied Maths, Sint-Martens-Latem, Belgium) was applied. PFGE patterns were compared to epidemic Swiss control strains. Control strain A was an epidemic strain in a single ward of the University Hospital of Basel in the year 2002.14 Control strain B was responsible for an outbreak on a geriatric ward in the north-western part of the country in the year 1992 A Widmer, (unpublished data). Control strain C was an epidemic strain having spread in the western part of Switzerland15 (kindly provided by DS Blanc, CHUV, Lausanne, Switzerland). The dendrograms were calculated by the unweighted pair group method using arithmetic averages (UPGMA).
Statistics
Categorized data were analyzed using the χ2-test or Fisher's exact test, where applicable and non-parametric data using the Mann–Whitney U-test.
Results
Epidemiological and clinical data
Among the 5992 admissions during the study period, 100 episodes of MRSA colonization (22 cases) or infection (78 cases) were reported in a total of 76 patients. For these patients, the mean duration of stay in hospital was 147 days (range: 8–634 days), compared to 63 days for MRSA-negative patients (P<0.001). Prolonged hospitalization for MRSA-positive patients was mainly due to a long isolation time (mean: 47.3 days), resulting in delayed or interrupted rehabilitation and to the high percentage of decubital ulcers (39%) on admission. Decubital ulcers were significantly more frequent (P<0.001) in MRSA-positive patients compared to the overall admitted MRSA-negative patients during the study period. Clinical data of the MRSA-positive patients are shown in Table 1. Among the MRSA-positive patients, male subjects were significantly (P=0.006) more frequent (82.9%) compared to the percentage of male subjects in overall admissions (68.2%), but traumatic injury among MRSA-positive male subjects (74.6%) was not more frequent compared to traumatic injury in MRSA-negative male subjects (74.8%). The mean rate of cases with MRSA positivity over the study period was 1.67 cases per 100 admissions (Figure 1). Of the 76 patients, 45 were admitted from Switzerland and 31 from foreign countries. Interestingly, among the admissions from abroad, 74.1% were community acquired, compared to 31% among admissions from Switzerland. Frequency of MRSA carriage at admission was strongly related to the country of origin. Patients admitted from Italian hospitals were more frequently colonized compared to patients transferred from Swiss hospitals (P<0.001).
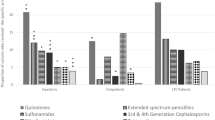
In 56% of MRSA-positive findings, MRSA was isolated in three or more body sites. Most frequent standard sites of localization were wounds, groin and nose (Table 2). Surprisingly, penis swabs were positive in 75% of all tested episodes. Penis swabs were only performed in case of urinary tract infection resistant to antibiotic treatment. None of the patients with positive penis swabs was circumcised.
Molecular typing
Pulsed-field gel electrophoresis was performed in isolates from 66 cultures of 60 patients or 78.9% of all patients. A total of 30 different strains were identified, most frequently strains A and C (Table 3). The strains of group A were collected over a time period of 5 years. Of these 11 cases, 5 were due to community acquisition. However, only two of the six remaining cases qualified for a nosocomial cluster. The 11 genetically identical strains of group C were isolated over a time period of 4½ years. Two nosocomial clusters could be distinguished, involving cases 1–3 (1996–1997) and cases 6–11 (1999–2000).
Overall four health-care workers (all not included in the statistics) were found MRSA positive; in two of them, the same strain as that found in patients was isolated. The remaining two, in whom epidemiological colonization through transmission from a patient truly could be excluded, were classified in groups H and X, respectively. Overall, a total of >1000 swabs were performed in health-care workers.
The 11 strains classified in groups B, G (Figure 2), and H were, except one, all community-acquired. Interestingly, four of them originated from Italy and four from the southern part of Switzerland (known regions of high MRSA prevalence). Both patients of group E were transferred almost at the same time from the same Swiss hospital, where an outbreak with the identical strain was documented. However, in one patient no screening at admission was performed; this case is thus subclassified as nosocomial (Table 3). Two strains (one of group F and one of group J) were classified as suspected nosocomial, because screening was performed between 48 and 96 h from admission; nevertheless, these cases possibly represent a community acquisition. The second patient of group K was still hospitalized at the closure of the study and is therefore not included. Among the 20 different cases summarized in group X, 10 were due to community acquisition and 8 of these due to admissions from abroad (Figure 3). Among the 10 strains classified as transferred, 4 were identified between 48 and 96 h after admission and therefore represent probably community-acquired cases. The nine cases of group O, which were identified beyond 48 h from admission, were classified as suspected nosocomial.
Successful decolonization program
Decolonization was successful in 60 of 76 (78.9%) MRSA-positive patients (Table 4), confirmed by three negative swab series. Considering the number of MRSA-positive episodes, 69 out of a total of 100 have been successfully decolonized. Figure 4 demonstrates the impact of the decolonization measures. The mean rate of MRSA positivity at discharge was 0.52 per 100 admissions, compared to admission data a significant improvement (P<0.001). Average MRSA negativity occurred after 44 days (range: 1–196 days). Among the 31 episodes with persistence of MRSA positivity (MRSA positive at discharge), 22 showed a high mean of MRSA-positive body sites (3.41, range: 2–6) already at admission. Pressure sores were with 14 times the most frequent site of persistent MRSA-positive localization, followed by urine (7 times). However, decolonization measures by conservative therapy or plastic surgery considerably reduced the total number of MRSA-infected pressure sores from 39 to 5 (87.2%). In particular, decubital ulcers were successfully treated by plastic surgery in 22 cases, whereas conservative therapy alone was successful in 12 cases.
Discussion
In a long-term prospective epidemiologic MRSA study performed at the SPC Nottwil, we were are able to demonstrate that rigorous infection control measures such as published in recently updated Society for Healthcare Epidemiology of America (SHEA) guidelines16 resulted in a significant reduction of MRSA incidence in SCI patients at the SPC Nottwil, comparing the MRSA-positive patients on admission and at discharge. Epidemiologic data on MRSA-positivity in Swiss SCI centers earlier to the observation period of our study are not available. MRSA colonization rates were very high in other (foreign) SCI centers, ranging up to over 70%.2 Some reduction of nosocomial infection rates and outbreaks of MRSA in SCI was achieved through the introduction of basic infection control measures,2 but MRSA represents still a threat in this group of patients, where it has recently been identified as an independent risk factor of nosocomial infection.17
Various earlier studies in non-SCI collectives demonstrated the efficacy of rigorous infection control measures to prevent intra-hospital spread of MRSA.9 In a recent study18 performed in a country with low MRSA incidence, even eradication of the pathogen has been reported. In Switzerland, where overall low rates of MRSA are still observed, endemic MRSA, present in southern and south-western regions, appears to result from continuous introduction of new strains from surrounding southern countries.11 Our national specialized center, where about 2% of the SCI patients are admitted from southern European countries, is confronted with high risk for MRSA introduction, since this small percentage of overall admissions made up for about 30% of our patients with community-acquired-MRSA positive infections.
We already in 1991 got aware of this emerging problem and therefore started an infection control program, including detailed clinical data collection and molecular MRSA typing. At the time of admission, screening of patients at risk identified nose, axilla, groin, wound, decubital ulcers and urinary tract as frequent sites of MRSA positivity, in accordance with earlier reports.2 Among non-standard screening sites, tracheobronchial secretion, pharynx, perianal region and penis, the latter not having been reported as to our knowledge, were most frequently colonized.
Our hygienic program, applying primary contact isolation procedures for patients admitted from regions with known high MRSA prevalence, is of prime importance. Since more than half of our cases were community acquired and consequently treated under isolation conditions from the beginning, further intra-hospital spread could be blocked. In only two instances, small endemic clusters of six and three patients, respectively, were identified over a 10-year period. This low incidence of nosocomial cases is probably due to the rigorous discipline of health-care workers concerned, because SCI patients, during early phases of the rehabilitation program, are unable to perform their intimate care themselves.
Decolonization of MRSA was possible in 60 (78.9%) of our SCI patients; beside the usual decolonization procedures and conservative therapy for decubital ulcers, plastic surgery probably plays a major role. In our experience, closure of decubital wounds by plastic surgery was very efficient in eradication of MRSA. However, SCI patients with several MRSA-positive body sites and extended decubital ulcers represent a problem still to be resolved. Thus, 5 of our 16 patients with persistent MRSA positivity left the clinic with a infected wound no more curable by plastic surgery.
Molecular typing of MRSA by PFGE is generally accepted as a standard method because of its reliability, inter-laboratory reproducibility and good correlation with epidemiologic data.19 Recently developed PCR-based approaches for molecular MRSA typing are faster and less expensive but have not yet shown superior discriminatory capacity compared to PFGE.14, 20 Using the PFGE technique in combination with detailed epidemiological anamnesis, we were able to identify two small MRSA outbreaks (nosocomial clusters) at our hospital. The founder strains of the two proven nosocomial clusters originated from primary rehabilitation patients admitted from Swiss places (Cantons of Aargau and Berne). Comparison with Swiss epidemiologic strains isolated over the same observation period14, 15 revealed no strain homology. Thus, vigorous application of the guidelines for prevention of nosocomial transmission limited intra-hospital spread to only nine patients in an SCI clinic over a 10-year period. The mean rate of MRSA positivity was significantly lower than the results of the recent International SENTRY study1 and also a Swiss national survey including university hospitals, community hospitals, long-term care and rehabilitation centers.11
In conclusion, this study provides strong evidence from the largest European SCI center that MRSA controlling and successful decolonization are feasible if infection control guidelines, such as recently published by SHEA,16 are vigorously applied.
Conflict of interest
None.
References
Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region of the SENTRY antimicrobial surveillance program, 1997–1999. Clin Infect Dis 2001; 32: S114–S132.
Thom JD, Wolfe V, Perkash I, Lin VW . Methicillin-resistant Staphylococcus aureus in patients with spinal cord injury. J Spinal Cord Med 1999; 22: 125–131.
Mylotte JM, Kahler L, Graham R, Young L, Goodnough S . Prospective surveillance for antibiotic-resistant organisms in patients with spinal cord injury admitted to an acute rehabilitation unit. Am J Infect Control 2000; 28: 291–297.
Crisostomo MI, Westh H, Tomasz A, Mk C, Oliveria DC, de Lencastre H . The evolution of methicillin resistance in Staphylococcus aureus: similarity of genetic backgrounds in historically early methicillin-susceptible and resistant and contemporary epidemic clones. Proc Natl Acad Sci USA 2001; 98: 9865–9870.
Vancomycin-resistant Staphylococcus aureus—Pennsylvania, 2002. MMWR Morb Mortal Wkly Rep 2002; 51: 902.
Charlson ME, Pompei P, Ales KL, MacKenzie CR . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383.
Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003; 26 (Suppl 1): S50–S56.
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM . CDC definitions for nosocomial infections. Am J Infect Control 1988; 16: 128–140.
Fluckiger U, Widmer AF . Epidemiology of methicillin-resistant Staphylococcus aureus. J Chemother 1999; 45: 121–134.
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH et al. Interpreting chromosomal DNA restriction pattern produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 1995; 33: 2233–2239.
Blanc DS, Pittet D, Ruef C, Widmer AF, Muhlemann K, Petignat C et al. Molecular epidemiology of predominant clones and sporadic strains of methicillin resistant Staphylococcus aureus in Switzerland and comparison with European epidemic clones. Clin Microbiol Infect 2002; 8: 419–426.
Jackson MM, Lynch P . Guideline for isolation precautions in hospitals. Am J Infect Control 1996; 24: 203–206.
National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests, 4th edn. Approved Standard. NCCLS document M2-A4. NCCLS: Villanova, PA, 1990.
Strandιn A, Frei R, Widmer AF . Molecular typing of methicillin-resistant Staphylococcus aureus: can PCR replace pulsed-field gel electrophoresis? J Clin Microbiol 2003; 41: 3181–3186.
Blanc DS, Petignat C, Moreillon P, Entenza JM, Eisenring M, Kleiber H et al. Unusual spread of a penicillin-susceptible methicillin resistant Staphylococcus aureus clone in a geographic area of low incidence. Clin Infect Dis 1999; 29: 1512–1518.
Muto CA, Jernigan CA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM et al. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 2003; 24: 362–386.
Mylotte JM, Graham R, Kahler L, Young R, Goodnough S . Epidemiology of nosocomial infection and resistant organisms in patients admitted for the first time to an acute rehabilitation unit. Clin Infect Dis 2000; 30: 425–432.
Kotilainen P, Routamaa M, Peltonen R, Evesti P, Eerola E, Salmenlinna S et al. Eradication of methicillin-resistant Staphylococcus aureus from a health center ward and associated nursing home. Arch Intern Med 2001; 161: 859–863.
Tenover FC, Arbeit R, Archer G, Biddle J, Byrne S, Goering R et al. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J Clin Microbiol 1994; 32: 407–415.
Deplano A, Schuermans A, Van Eldere J, Witte W, Meugnier H, Etienne J et al. Multicenter evaluation of epidemiological typing of methicillin-resistant Staphylococcus aureus strains by repetitive-element PCR analysis. J Clin Microbiol 2000; 38: 3527–3533.
Acknowledgements
This study was supported by the Swiss Paraplegic Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kappel, C., Widmer, A., Geng, V. et al. Successful control of methicillin-resistant Staphylococcus aureus in a spinal cord injury center: a 10-year prospective study including molecular typing. Spinal Cord 46, 438–444 (2008). https://doi.org/10.1038/sj.sc.3102135
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3102135