Abstract
Study design:
Case–control study.
Aim of the study:
Investigate the presence of additional endogenous risk factors of deep-vein thrombosis (DVT).
Setting:
Regional Spinal Unit of Florence, Italy.
Methods:
A total of 43 patients with spinal lesion and a history of DVT during the acute stage of their neurological impairment (Group A) were comprehensively evaluated and the blood concentrations of the following risk factors, that are presumably associated with DVT, were determined: antithrombin III (ATIII), protein C (PC), protein S (PS), factor V Leiden, gene 200210A polymorphism, homocysteine (Hcy), inhibitor of plasminogen activator-1 (PAI-1) and lipoprotein A (LpA). The control group (Group B) consisted of 46 patients matched to Group A for sex, age, neurological status and prophylactic treatment during the acute stage, with no history of DVT. Statistical analysis was performed using the Mann–Whitney and Fisher's exact tests.
Results:
Of the individuals in Group A, 14% had no risk factor and 86% had at least one; however, in Group B 54% had no endogenous risk factors and 46% had at least one. None of the individuals in either group had a deficit in their coagulation inhibitors (ATIII, PC and PS), and the LpA level was equivalent in the two groups. The levels of Hcy and PAI-1 were significantly higher in Group A.
Conclusions:
Increases in the levels of plasma Hcy and PAI-1 are demonstrated to be independent risk factors for developing a DVT.
Similar content being viewed by others
Introduction
Deep-vein thrombosis (DVT) is one of the most common and highly feared events that occurs as a complication of acute spinal cord lesion (SCL), as well documented in the literature.1, 2, 3, 4, 7 The reported incidence ranges from 9 to 90%, depending on the research methods used, the nature of the study population under investigation, and the prophylactic strategy that was adopted. Nevertheless, in more recent and widespread studies, it is reported to vary between 10 and 30%.1, 2, 3, 5, 6 It is well-known that in acute SCI, all the factors that characterize the Virchow triad occur: the first risk factor in SCI is stasis of circulating blood: acute palsy leads to loss of the muscle pump, dilatation of the blood vessels, and decreased blood flow from the lower limbs. The second risk factor for DVT is hypercoagulability: some changes in hemostasis have been observed in SCI patients before the thrombotic event, including increases in Factor VIII, and increased levels and aggregation of platelets.8 The third risk factor that has been identified is damage to venous endothelial cells.
Furthermore, several clinical conditions and diseases are considered to be acquired risk factors, including immobilization, pregnancy, chronic hearth failure, varicose veins, age, and obesity, all of which can increase venous stasis. On the other hand, clinical conditions such as trauma, surgery (eg, orthopedics, neurosurgery), use of central vein catheters, tumors, myocardial infarction, hematological diseases (leukemia, thrombocytosis, etc), and use of estrogens, are all risk factors for hypercoagulation.9, 10
There are many congenital risk factors that may also play an important role in the development of a DVT, each one associated with an increased thrombophylic risk. The following have been identified as possible candidates: hereditary antithrombin III (ATIII) deficiency, hereditary protein C (PC) deficiency, hereditary protein S (PS) deficiency, activated PC resistance (factor V Leiden), plasminogen activator disorders, gene 20210A polymorphism (the codifying gene for prothrombin), inhibitor of plasminogen activator I (PAI-1) disorders, increased levels of lipoprotein A (LpA), the presence of antiphospholipid antibodies, and hyperhomocysteinemia (congenital and acquired).9
ATIII is the most potent inhibitor of the coagulation cascade, exerting some level of inhibitory activity on virtually all of the coagulation enzymes. However, the primary targets that ATIII inhibits are factor Xa, factor IXa, and thrombin (factor IIa). Nonetheless, it also has some ability to inhibit factor XIIa, factor XIa, and the complex of factor VIIa and tissue factor. The ability of ATIII to limit coagulation through multiple interactions makes it one of the primary natural anticoagulant proteins.9, 10
PC inactivates factor Va and factor VIIIa by converting them to their inactive forms (factor V and factor VIII, respectively). There are several congenital risk factors that are associated with loss of the activity of PC. Deficiency of PC itself is a genetic trait that predisposes one to the formation of venous clots. In addition, PS serves as a cofactor with PC in the inactivation of factor Va and factor VIIIa. Deficiency of PS therefore prevents the activity of PC.9, 10
PAI-1 is a member of a family of proteins that inhibits plasminogen activators.11 PAI-1 is a single-chain glycoprotein. During fibrinolysis, tissue plasminogen activator (tPA) converts the inactive protein plasminogen into plasmin. Plasmin, in turn, plays a critical role in fibrinolysis by degrading fibrin. PAI-1 limits the production of plasmin and serves to keep fibrinolysis in check. PAI-1 levels are, in part, controlled on a genetic basis. Certain polymorphisms in the PAI-1 gene are associated with increased blood concentrations.11
Prothrombin polymorphism G20210A and factor V Leiden: these two polymorphisms were found to be associated with an increased risk for venous thromboembolism. Factor V Leiden confers a hypercoagulable state as it determines the synthesis of a modified factor V which shows an ‘in vitro’ resistance to the inhibitory action of PC. Its prevalence ranges from 3 to 5% in the Western population. Moreover, the G20210A polymorphism in the gene coding for prothrombin, whose prevalence ranges from 2 to 3% in the Western population, is associated with a significantly higher risk of venous thromboembolism, but the mechanism underlying this hypercoagulable effect is not well defined. An hypothesis is based on studies which have documented that the subjects carrying such polymorphism have higher circulating levels of prothrombin.9, 12, 13
Lp(a) consists of a particle of low-density lipoprotein cholesterol (LDL-C) linked by a disulfide bond to a large hepatically derived glycoprotein, apolipoprotein (a), which is structurally similar to plasminogen. Although the mechanism through which Lp(a) promotes cardiovascular disease is yet to be elucidated, there are two possible hypotheses for this activity:
-
1
the apolipoprotein (a) moiety could promote thrombogenesis and/or
-
2
its LDL-C moiety could promote atherogenesis.
The apolipoprotein portion of Lp(a) competitively displaces plasminogen from binding sites on both fibrin and endothelial cells.14 Lp(a) is also associated with increased levels of PAI-1 and decreased activity of t-PA.15 These effects all combine to promote thrombosis and inhibit fibrinolysis. The physiological role of Lp(a) is unknown: levels are largely genetically determined, increase slightly with age, and vary by race. Elevated levels of Lp(a) are found in patients with chronic renal failure, nephrotic syndrome, and diabetic nephropathy.16, 17
Homocysteine (Hcy) is derived from the essential amino acid methionine. The levels of Hcy in the blood depend on the rate of its synthesis by an extensive enzymatic pathway, as well as the levels of vitamin B12, folate, and 5-methyltetrahydrofolate (produced by methylenetetrahydrofolate reductase).18 Mild elevations in the plasma levels of Hcy result from folate and vitamin B12 deficiency and renal diseases. These elevations in Hcy are associated with several common diseases, including cardiovascular diseases. The accumulation of Hcy leads to increased cellular oxidative stress, and chronic exposure to Hcy causes endothelial and vascular dysfunction.9, 10
Aim of our study was to better determine the thrombophylic risk profile of patients during the early phase of spinal lesion. In order to achieve this, we evaluated the levels of the above-mentioned endogenous factors that have previously been associated with congenital disorders. By comparing the levels of these endogenous factors in the blood of patients, with or without a DVT, we will be able to determine whether they are risk factors.
Patients and methods
We assessed the blood levels of the following endogenous risk factors of 43 patients (Group A) with a SCL, previously admitted to the Spinal Unit of Florence (from July 1999 till February 2004), and with a history of DVT during the acute stage (the first 30 days) after the lesion: ATIII, PC, PS, factor V Leiden, gene 20210A polymorphism (the codifying gene for prothrombin), homocysteine, inhibitor of PAI-1, and LpA. DVT had occurred in all patients during the first month following the lesion and they were detected using an Ultrasound echo–color–doppler. The blood examinations, aimed to measure the concentration of the above-mentioned factors, were assessed at least 12 months after the lesion. None of them was under pharmacological antithrombotic treatment during the performance of the blood tests.
Group A patients were compared to 46 patients (Group B or control group) who had been admitted to the Spinal Unit of Florence in the same time period, with no history of DVT. The characteristics of the patients in both groups were homogeneous with regard to gender, mean age, prophylaxis for DVT, neurological lesion level, and the ASIA/ISCOS impairment scale.19 All the patients completed a detailed survey in order to determine their familial history of DVT.
Inclusion criteria
Patients with SCL admitted to our center from July 1999 till February 2004 were included in the study: all of them agreed and signed the consent form.
Exclusion criteria
To further minimize variation between the two groups, we excluded patients who had undergone pneumatic compression to the lower extremities. Although pneumatic compression to the lower extremities is part of our prophylaxis protocol for DVT, those patients who had DVT at the time of admission to the unit and those admitted for more than 3 weeks following the lesion did not undergo such a procedure, and therefore were able to be included in the study. Anyway, all patients had been treated with subcutaneous low-molecular weight heparin as pharmacological prophylaxis for DVT.
Experimental procedures
Venous blood was collected between 8 and 10 am, following fasting overnight. The first 2 ml of blood was discarded and blood samples were drawn in evacuated tubes (Vacutainer, Becton Dickinson, Meylan, France) containing 0.129 mol/l sodium citrate (final ratio with blood 1/10) (for AT, PC, PS, and PAI-1), ethylenediaminetetraacetic acid (for Hcy), and no anticoagulant (for Lp(a)). Blood samples were preserved and centrifuged at 4°C (for measurement of PAI-1 and Hcy) or 18°C (for measurement of AT, PC, PS, and Lp(a)), at 2000 r.p.m. for 10 min, snap frozen in liquid nitrogen and stored at −80°C. The aliquots were then thawed and assayed for the levels of the endogenous factors within 7 days. The activities of AT and PC were evaluated by automated chromogenic methods (DADE, Behring, Marnurg, Germany). The levels of free PS (Asserachrom, Diagnostica Stago, Asnieres sur Seine, France), and the activities of PAI-1 (PAI-1 Asserachrom, Diagnostica Stago, Asnieres sur Seine, France) and Lp(a) (Apo(a) ELISA, Mercordia), were measured by enzyme-linked immunosorbent assay. The levels of Hcy were measured by immunoassay (FPIA assay, IMx system, Abbott, USA).
Presence of the Factor V Leiden allele and the G20210A polymorphism in the factor II gene were detected by extracting genomic DNA from peripheral blood leukocytes using a MagNA Pure (Roche) and QUIAmp Blood Kit (QUIAGEN, Hilden, Germany), respectively. Factor V Leiden and G20210A polymorphism in the factor II gene were identified using the light-cycler capillaries method, as per manufacturer's instructions (Roche).
Statistical analysis
Unless otherwise indicated, the median result is given (range). The nonparametric Mann–Whitney test for unpaired data was used for comparisons between the single groups. Univariate and multivariate analyses (adjusted for age, sex, and acquired risk factors) were used to determine the risk associated with elevated levels of PAI-1, Lp(a), Hcy levels, and heterozygosity for Factor V Leiden and Factor II polymorphism. Deficiencies of AT, PC, and PS were not included in logistic regression because no alterations were found to the levels of these proteins in any of the patients. Levels of Lp(a) above 300 mg/l, a level that is associated with an increased risk of occlusive arterial disease, were considered over the normal range. Hcy and PAI-1 were considered to be elevated when plasma levels exceeded the 95th percentile of the distribution of values obtained in controls (Hcy: 13 μmol/l in females ad 19 μmol/l in males; PAI-1: 40 mg/dl). All odds ratios (OR) are given with a 95% confidence interval (CI). All probability values are two–tailed, with values of less than 0.05 considered statistically significant.
Results
Of the 89 patients studied, 71 were male and 18 were female, with a mean age of 50.5 years (range 21–82). The causes of the SCL were traumatic in 74 patients and not traumatic in 15. Of the traumatic injuries, 38 were the result of road traffic accidents, 24 of falls, eight of sports-related accidents, two of gun wounds, and two of other causes. The nontraumatic cases were owing to vascular malformation (three cases), spinal cord compression (three cases), myelitis (two cases), and other causes (seven cases). Of the whole group, 62 were paraplegic and 27 were tetraplegic, with impairments distributed along the ASIA scale as follows: A=52 cases, B=16 cases, C=9 cases, and D=12 cases.
Group A comprised 43 patients, of whom 34 were male and 9 were female, with a mean age of 50 years (range 13–78). Thirty-two were paraplegic and 11 were tetraplegic, with impairments distributed along the ASIA scale as follows: A=28 cases, B=7 cases, C=3 cases, and D=5 cases. They represent 13% of all admitted acute patients during the same time period and the majority of cases of DVT detected during that stage. As stated before, they were all admitted to our Unit at least 3 weeks after the lesion and had received the same pharmacological prophylaxis for DVT in other departments or hospitals.
Group B (control group) was composed of 46 patients, of whom 35 were male and 11 were female, with a mean age of 50.5 years (range 21–82). Thirty-one were paraplegic and 15 were tetraplegic, with impairments distributed along the ASIA scale as follows: A=24 cases, B=9 cases, C=6 cases, and D=7 cases.
They also received the same pharmacological prophylaxis and did not undergo sequential pneumatic compression to the lower extremities as well. They represent 14% of all admitted acute patients during the same period.
Group A vs Group B: in Group A, 14% of the individuals had none of the endogenous risk factors, whereas 86% had at least one. In Group B, 54% had none of the endogenous risk factors, whereas 46% had at least one. None of the patients from either the groups had a defect in the physiological inhibitors of coagulation (ATIII, PC and S). In addition, the levels of Lp(a) were similar between the two groups (Group A: 104 (1–487) mg/l; Group B: 84.5 (1–1079) mg/l) (Table 1).
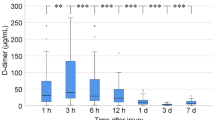
However, the mean plasma concentrations of Hcy and of PAI-1 were significantly higher in Group A than in Group B (P<0.05 for both) (Figure 1 and Table 1).
We used multivariate analysis to investigate if the association between the two endogenous risk factors (Homocysteine and PAI-1) and DVT could even occur together with other acquired risk factors. Our results (Figure 1):
-
plasma hyperhomocysteinemia: OR=3.6 (95% CI 1.4–9.5) P<0.007
-
high level of PAI-1: OR=4.9 (95% CI 1.7–14) P<0.002
Factor V Leiden was found in three patients (two of Group A and one of Group B; P=0.474); prothrombin polymorphism was documented in two patients (one of Group A and one of Group B; P=0736).
Discussion and conclusions
DVT is one of the most common and highly feared events that occurs as a complication of acute SCL because all the factors that characterize the Virchow triad occur. Investigating the presence of additional endogenous risk factors of DVT, that can predispose to hypercoagulability, was the purpose of our study.
Our data demonstrate that hyperhomocysteinemia and impaired fibrinolysis, indicated by high levels of its main inhibitor PAI-1, are independent risk factors for the occurrence of DVT in patients with SCL. This is therefore the first clear demonstration that there is a prevalence of particular thrombophylic risk factors in patients with SCL with a positive history of DVT in the acute stage.
Thus far, no data have been published describing Hcy levels in patients with DVT after SCL. Hcy is an aminoacid whose role as a risk factor for arterial and venous thrombosis has been well documented, although the available data regarding the effects of pharmacological correction are conflicting. High levels of Hcy occur as a result of both genetic and environmental factors. Such elevations have been demonstrated to occur in individuals with the C677T polymorphism in the gene coding for methyltetrahydrofolatereductase.20 In addition, low levels of vitamins that act as cofactors for the enzymes that are involved in the synthesis of Hcy, that is, folic acid, vitamin B6, and vitamin B12, is the other main cause of hyperhomocysteinemia.18 Interestingly, it has recently been demonstrated that deficiencies of folic acid and vitamin B6 are thrombotic risk factors, independent of the levels of Hcy.21, 22 Our results reveal a possible therapeutic target, as the levels of Hcy can be lowered using vitamin supplementation based on folic acid, vitamin B6, and B12.18
The demonstration of an effect of PAI-1 levels underlines the role of fibrinolysis in SCL patients with DVT. Elevated PAI-1 levels result in impaired fibrinolysis, and have long been reported to be a risk factor for arterial and venous thrombosis.23, 24 Furthermore, elevated levels of PAI-1 are related to the presence of dyslipidemia (both hypercholesterolemia and hypertriglyceridemia), low physical activity, and high body mass index.25 In our study, there was a significantly greater prevalence of elevated PAI-1 in SCI patients, but a multivariate analysis nonetheless demonstrated that it was a risk factor for a previous DVT. Again, these results identify a target that is potentially amenable to therapeutic intervention.
Previous intervention trials have explored the use of statins and suggested a benefit of this class of drugs, not only in reducing the lipid plasma levels of PAI-1 but also on the fibrinolytic system itself.25
No data have previously been published on the possible role of thrombophylic polymorphism in these patients. Our results do not indicate a possible role for these alterations, as we found that their prevalence was similar in patients with and without a previous DVT. However, the patient groups we studied were insufficient to allow us to draw definitive conclusion on the prevalence of genetic polymorphism.
The following limitations should be considered with respect to the conclusions of this study. Firstly, the levels of some of the analytes, in particular Hcy, PAI-1, and Lp(a), may have been influenced by transient states, that is inflammatory processes for Lp(a) and PAI-1 levels or changes in dietary habits for Hcy. For this reason, data on these parameters should be treated with caution. Secondly, the retrospective design of this study prevents determination of any causal link between the hemostatic parameters and the previous occurrence of DVT.
We have demonstrated that Hcy and PAI-1 are risk factors for patients with an SCI who have previously had a DVT. Both of these endogenous factors are susceptible to pharmacological intervention. We therefore plan to prospectively investigate Hcy and PAI-1 levels in all new admitted patients, to help us better define such risk factors and to assess the efficacy of an additional prophylaxis, such as vitamin supplementation and/or a statin.
References
Kulkarni JR, Burt AA, Tromans AT, Constable PD . Prophylactic low dose heparin anticoagulant therapy in patients with spinal cord injuries: a retrospective study. Paraplegia 1992; 30: 169–172.
Waring WP, Karunas RS . Acute spinal cord injuries and the incidence of clinically occurring thromboembolic disease. Paraplegia 1991; 29: 8–16.
Merli GJ et al. Aetiology, incidence, and prevention of deep vein thrombosis in acute spinal cord injury. Arch Phys Med Rehabil 1993; 74: 1199–1205.
Aito S, Pieri A, D'Andrea M, Marcelli F, Cominelli E . Primary prevention of deep venous thrombosis and pulmonary embolism in acute spinal cord injured patients. Spinal Cord 2002; 40: 300–303.
Yelnik A et al. Systematic lower limb phlebography in acute spinal cord injury in 147 patients. Paraplegia 1991; 29: 253–260.
Powell M, Kirschblum S, O'Connor KC . Duplex ultrasound screening for deep vein thrombosis in spinal cord injured patients at rehabilitation admission. Arch Phys Med Rehabil 1999; 80: 1044–1046.
Gunduz S et al. Deep vein thrombosis in spinal cord injured patients. Paraplegia 1993; 31: 606–610.
Rossi E, Green D, Rosen J . Sequential changes in factor VIII and platelets preceding deep vein thrombosis in patients with spinal cord injury. Br J Haematol 1980; 45: 143–151.
Lopez JA, Kearon C, Lee AY . Deep venous thrombosis. Hematology (Am Soc Hematol Educ Program) 2004; 439–456 Review.
De Stefano V, Finazzi G . Mannucci, inherited thrombophilia: pathogenesis clinical syndromes management. Blood 1996; 87: 3531–3544.
Huber K et al. Plasminogen activator inhibitor type-1 in cardiovascular disease, status report 2001. Thromb Res 2001; 103 (Suppl 1): S7–S19.
De Stefano V, Rossi E, Paciaroni K, Leone G . Screening for inherited thrombophilia: indications and therapeutic implications. Haematologica 2002; 87: 1095–1108.
Price DT, Ridker PM . Factor V Leiden mutation and the risks for thromboembolic disease: a clinical perspective. Ann Intern Med 1997; 127: 895–903.
Loscalzo J et al. Lipoprotein(a), fibrin binding, and plasminogen activation. Arteriosclerosis 1990; 10: 240–245.
Stein JH, Rosenson RS . Lipoprotein Lp(a) excess and coronary heart disease. Arch Intern Med 1997; 157: 1170–1176.
Cressman MD et al. Lipoprotein(a) is an independent risk factor for cardiovascular disease in hemodialysis patients. Circulation 1992; 86: 475–482.
Wanner C et al. Elevated plasma lipoprotein(a) in patients with the nephrotic syndrome. Ann Intern Med 1993; 119: 263–269.
Selhub J . Homocysteine metabolism. Annu Rev Nutr 1999; 19: 217–246.
Marino RJ et al. ASIA neurological standards committee 2002. International standards for neurological classification of spinal cord injury. J Spinal Cord Med 2003; 26: S50–S56.
Abbate R et al. The high prevalence of thermolabile 5–10 methylenetetrahydrofolate reductase (MTHFR) in Italians is not associated to an increased risk for coronary artery disease (CAD). Thromb Haemost 1998; 79: 727–730.
Cattaneo M, Lombardi R, Lecchi A, Bucciarelli P, Mannucci PM . Low plasma levels of vitamin B(6) are independently associated with a heightened risk of deep-vein thrombosis. Circulation 2001; 104: 2442–2446.
Robinson K et al. Low circulating folate and vitamin B6 concentrations: risk factors for stroke, peripheral vascular disease, and coronary artery disease. European COMAC Group. Circulation 1998; 97: 437–443.
Kohler HP, Grant PJ . Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med 2000; 342: 1792–1801.
Crowther MA et al. Fibrinolytic variables in patients with recurrent venous thrombosis: a prospective cohort study. Thromb Haemost 2001; 85: 390–394.
Kaba NK et al., THROMBO Investigators. Effects of lipids and lipid-lowering therapy on hemostatic factors in patients with myocardial infarction. J Thromb Haemost 2004; 2: 718–725.
Acknowledgements
We thank Loredana Ricci, MD, for her precious contribution in collecting data.This is a spontaneous research, not funded or financed by any institution or private company.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aito, S., Abbate, R., Marcucci, R. et al. Endogenous risk factors for deep-vein thrombosis in patients with acute spinal cord injuries. Spinal Cord 45, 627–631 (2007). https://doi.org/10.1038/sj.sc.3102018
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.sc.3102018
Keywords
This article is cited by
-
Against all odds: a qualitative study of rehabilitation of persons with spinal cord injury in Afghanistan
Spinal Cord (2012)
-
Central cord syndrome in Ireland: the effect of age on clinical outcome
European Spine Journal (2009)
-
The prevalence of deep vein thrombosis in a cohort of patients with spinal cord injury following the Pakistan earthquake of October 2005
Spinal Cord (2008)